RAGE-dependent potentiation of TRPV1 currents in sensory neurons exposed to high glucose
- PMID: 29474476
- PMCID: PMC5825096
- DOI: 10.1371/journal.pone.0193312
RAGE-dependent potentiation of TRPV1 currents in sensory neurons exposed to high glucose
Abstract
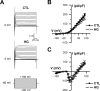
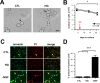
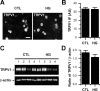
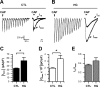
Diabetes mellitus is associated with sensory abnormalities, including exacerbated responses to painful (hyperalgesia) or non-painful (allodynia) stimuli. These abnormalities are symptoms of diabetic peripheral neuropathy (DPN), which is the most common complication that affects approximately 50% of diabetic patients. Yet, the underlying mechanisms linking hyperglycemia and symptoms of DPN remain poorly understood. The transient receptor potential vanilloid 1 (TRPV1) channel plays a central role in such sensory abnormalities and shows elevated expression levels in animal models of diabetes. Here, we investigated the function of TRPV1 channels in sensory neurons cultured from the dorsal root ganglion (DRG) of neonatal mice, under control (5mM) and high glucose (25mM) conditions. After maintaining DRG neurons in high glucose for 1 week, we observed a significant increase in capsaicin (CAP)-evoked currents and CAP-evoked depolarizations, independent of TRPV1 channel expression. These functional changes were largely dependent on the expression of the receptor for Advanced Glycation End-products (RAGE), calcium influx, cytoplasmic ROS accumulation, PKC, and Src kinase activity. Like cultured neurons from neonates, mature neurons from adult mice also displayed a similar potentiation of CAP-evoked currents in the high glucose condition. Taken together, our data demonstrate that under the diabetic condition, DRG neurons are directly affected by elevated levels of glucose, independent of vascular or glial signals, and dependent on RAGE expression. These early cellular and molecular changes to sensory neurons in vitro are potential mechanisms that might contribute to sensory abnormalities that can occur in the very early stages of diabetes.
Conflict of interest statement
Figures





References
-
- Spruce MC, Potter J, Coppini D V. The pathogenesis and management of painful diabetic neuropathy: a review. Diabet Med. 2003;20: 88–98. - PubMed
-
- Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta—Mol Basis Dis. Elsevier B.V.; 2009;1792: 931–940. doi: 10.1016/j.bbadis.2008.11.005 - DOI - PubMed
-
- Kamei J, Zushida K, Morita K, Sasaki M, Tanaka S. Role of vanilloid VR1 receptor in thermal allodynia and hyperalgesia in diabetic mice. Eur J Pharmacol. 2001;422: 83–6. - PubMed
-
- Khan GM, Chen S-R, Pan H-L. Role of primary afferent nerves in allodynia caused by diabetic neuropathy in rats. Neuroscience. 2002;114: 291–9. - PubMed
-
- Rashid MH, Inoue M, Bakoshi S, Ueda H. Increased Expression of Vanilloid Receptor 1 on Myelinated Primary Afferent Neurons Contributes to the Antihyperalgesic Effect of Capsaicin Cream in Diabetic Neuropathic Pain in Mice. J Pharmacol Exp Ther. 2003;306. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

