Insect tissue-specific vitellogenin facilitates transmission of plant virus
- PMID: 29474489
- PMCID: PMC5849359
- DOI: 10.1371/journal.ppat.1006909
Insect tissue-specific vitellogenin facilitates transmission of plant virus
Abstract
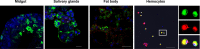
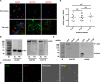
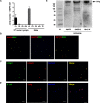
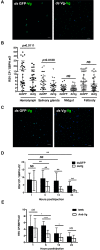
Insect vitellogenin (Vg) has been considered to be synthesized in the fat body. Here, we found that abundant Vg protein is synthesized in Laodelphax striatellus hemocytes as well. We also determined that only the hemocyte-produced Vg binds to Rice stripe virus (RSV) in vivo. Examination of the subunit composition of L. striatellus Vg (LsVg) revealed that LsVg was processed differently after its expression in different tissues. The LsVg subunit able to bind to RSV exist stably only in hemocytes, while fat body-produced LsVg lacks the RSV-interacting subunit. Nymph and male L. striatellus individuals also synthesize Vg but only in hemocytes, and the proteins co-localize with RSV. We observed that knockdown of LsVg transcripts by RNA interference decreased the RSV titer in the hemolymph, and thus interfered with systemic virus infection. Our results reveal the sex-independent expression and tissue-specific processing of LsVg and also unprecedentedly connect the function of this protein in mediating virus transmission to its particular molecular forms existing in tissues previously known as non-Vg producing.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Triazophos-induced vertical transmission of rice stripe virus is associated with host vitellogenin in the small brown planthopper Laodelphax striatellus.Pest Manag Sci. 2020 May;76(5):1949-1957. doi: 10.1002/ps.5729. Epub 2020 Jan 10. Pest Manag Sci. 2020. PMID: 31858699
-
Transovarial transmission of a plant virus is mediated by vitellogenin of its insect vector.PLoS Pathog. 2014 Mar 6;10(3):e1003949. doi: 10.1371/journal.ppat.1003949. eCollection 2014 Mar. PLoS Pathog. 2014. PMID: 24603905 Free PMC article.
-
Functional analyses of dopamine receptors involved in virus transmission and reproduction in the small brown planthopper Laodelphax striatellus.Pestic Biochem Physiol. 2024 Nov;205:106157. doi: 10.1016/j.pestbp.2024.106157. Epub 2024 Oct 1. Pestic Biochem Physiol. 2024. PMID: 39477610
-
The small brown planthopper (Laodelphax striatellus) as a vector of the rice stripe virus.Arch Insect Biochem Physiol. 2023 Feb;112(2):e21992. doi: 10.1002/arch.21992. Epub 2022 Dec 27. Arch Insect Biochem Physiol. 2023. PMID: 36575628 Review.
-
New insights on the transmission mechanism of tenuiviruses by their vector insects.Curr Opin Virol. 2018 Dec;33:13-17. doi: 10.1016/j.coviro.2018.07.004. Epub 2018 Jul 18. Curr Opin Virol. 2018. PMID: 30029017 Review.
Cited by
-
GrpE is involved in mitochondrial function and is an effective target for RNAi-mediated pest and arbovirus control.Insect Mol Biol. 2022 Jun;31(3):377-390. doi: 10.1111/imb.12766. Epub 2022 Feb 24. Insect Mol Biol. 2022. PMID: 35141960 Free PMC article.
-
The low-density lipoprotein receptor and apolipoprotein E associated with CCHFV particles mediate CCHFV entry into cells.Nat Commun. 2024 May 28;15(1):4542. doi: 10.1038/s41467-024-48989-5. Nat Commun. 2024. PMID: 38806525 Free PMC article.
-
Regulatory Mechanisms of Vitellogenesis in Insects.Front Cell Dev Biol. 2021 Jan 28;8:593613. doi: 10.3389/fcell.2020.593613. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33634094 Free PMC article. Review.
-
Microsporidian Nosema bombycis hijacks host vitellogenin and restructures ovariole cells for transovarial transmission.PLoS Pathog. 2023 Dec 7;19(12):e1011859. doi: 10.1371/journal.ppat.1011859. eCollection 2023 Dec. PLoS Pathog. 2023. PMID: 38060601 Free PMC article.
-
Gut-Expressed Vitellogenin Facilitates the Movement of a Plant Virus across the Midgut Wall in Its Insect Vector.mSystems. 2021 Jun 29;6(3):e0058121. doi: 10.1128/mSystems.00581-21. Epub 2021 Jun 8. mSystems. 2021. PMID: 34100642 Free PMC article.
References
-
- Hibino H. Biology and epidemiology of rice viruses. Annu Rev Phytopathol. 1996;34:249–74. doi: 10.1146/annurev.phyto.34.1.249 - DOI - PubMed
-
- Wang HD, Chen JP, Zhang HM, Sun XL, Zhu JL, Wang AG, et al. Recent Rice stripe virus epidemics in Zhejiang province, China, and experiments on sowing date, disease-yield loss relationships, and seedling susceptibility. Plant Dis. 2008;92(8):1190–6. doi: 10.1094/Pdis-92-8-1190 PMID: WOS:000257935700008 - DOI - PubMed
-
- Zhou YJ, Li S, Cheng ZB, Zhou T, Fan YJ. Research advances in rice stripe disease in China. Jiangsu J Agric Sci. 2012;28:1007–15
-
- Falk BW, Tsai JH. Biology and molecular biology of viruses in the genus Tenuivirus. Annu Rev Phytopathol. 1998;36:139–63. doi: 10.1146/annurev.phyto.36.1.139 - DOI - PubMed
-
- Huo Y, Liu WW, Zhang FJ, Chen XY, Li L, Liu QF, et al. Transovarial transmission of a plant virus is mediated by vitellogenin of its insect vector. PLoS Pathog. 2014;10(3). doi: ARTN e1003949 doi: 10.1371/journal.ppat.1003949 PMID: WOS:000337470300017. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

