Validation of the portable Air-Smart Spirometer
- PMID: 29474502
- PMCID: PMC5825056
- DOI: 10.1371/journal.pone.0192789
Validation of the portable Air-Smart Spirometer
Abstract
Background: The Air-Smart Spirometer is the first portable device accepted by the European Community (EC) that performs spirometric measurements by a turbine mechanism and displays the results on a smartphone or a tablet.
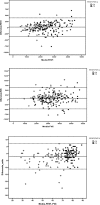
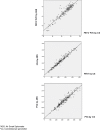
Methods: In this multicenter, descriptive and cross-sectional prospective study carried out in 2 hospital centers, we compare FEV1, FVC, FEV1/FVC ratio measured with the Air Smart-Spirometer device and a conventional spirometer, and analyze the ability of this new portable device to detect obstructions. Patients were included for 2 consecutive months. We calculate sensitivity, specificity, positive and negative predictive value (PPV and NPV) and likelihood ratio (LR +, LR-) as well as the Kappa Index to evaluate the concordance between the two devices for the detection of obstruction. The agreement and relation between the values of FEV1 and FVC in absolute value and the FEV1/FVC ratio measured by both devices were analyzed by calculating the intraclass correlation coefficient (ICC) and the Pearson correlation coefficient (r) respectively.
Results: 200 patients (100 from each center) were included with a mean age of 57 (± 14) years, 110 were men (55%). Obstruction was detected by conventional spirometry in 73 patients (40.1%). Using a FEV1/FVC ratio smaller than 0.7 to detect obstruction with the Air Smart-Spirometer, the kappa index was 0.88, sensitivity (90.4%), specificity (97.2%), PPV (95.7%), NPV (93.7%), positive likelihood ratio (32.29), and negative likelihood ratio (0.10). The ICC and r between FEV1, FVC, and FEV1 / FVC ratio measured by the Air Smart Spirometer and the conventional spirometer were all higher than 0.94.
Conclusion: The Air-Smart Spirometer is a simple and very precise instrument for detecting obstructive airway diseases. It is easy to use, which could make it especially useful non-specialized care and in other areas.
Conflict of interest statement
Figures
References
-
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med.20171;195(5):557–582. doi: 10.1164/rccm.201701-0218PP - DOI - PubMed
-
- Becker AB, Abrams EM. Asthma guidelines: the Global Initiative for Asthma in relation to national guidelines. Curr Opin Allergy Clin Immunol.2017;17(2):99–103. doi: 10.1097/ACI.0000000000000346 - DOI - PubMed
-
- Represas-Represas C, Botana-Rial M, Leiro-Fernández V, González-Silva A., García-Martínez A, Fernández-Villar A. Short- and Long-Term Effectiveness of a Supervised Training Program in Spirometry Use for Primary Care Professionals. Arch Bronconeumol.2013;49:378–382. doi: 10.1016/j.arbres.2013.01.001 - DOI - PubMed
-
- De Miguel-Díez J, Izquierdo-Alonso J.L, Rodríguez-González-Moro J.M, Lucas-Ramos P.D, Bellón-Cano J.M, Molina-Paris J. Reliability of chronic obstructive pulmonary disease diagnosis by primary care physicians and pneumologists in Spain. Predictive factors. Arch Bronconeumol.2004;40:431–7. - PubMed
-
- Represas-Represas C, Botana-Rial M, Leiro-Fernández V, Gonzalez Silva A, Del Campo Pérez V, Fernández Villar A. Validación del dispositivo portátil COPD-6 para la detección de patologías obstructivas de la vía aérea. Arch Bronconeumol.2010;46:426–432. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources