Modulation of Phase Shift between Wnt and Notch Signaling Oscillations Controls Mesoderm Segmentation
- PMID: 29474908
- PMCID: PMC5847172
- DOI: 10.1016/j.cell.2018.01.026
Modulation of Phase Shift between Wnt and Notch Signaling Oscillations Controls Mesoderm Segmentation
Abstract
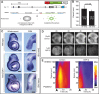
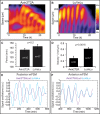
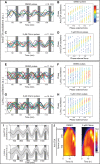
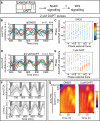
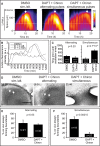
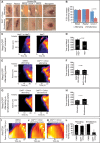
How signaling dynamics encode information is a central question in biology. During vertebrate development, dynamic Notch signaling oscillations control segmentation of the presomitic mesoderm (PSM). In mouse embryos, this molecular clock comprises signaling oscillations of several pathways, i.e., Notch, Wnt, and FGF signaling. Here, we directly address the role of the relative timing between Wnt and Notch signaling oscillations during PSM patterning. To this end, we developed a new experimental strategy using microfluidics-based entrainment that enables specific control of the rhythm of segmentation clock oscillations. Using this approach, we find that Wnt and Notch signaling are coupled at the level of their oscillation dynamics. Furthermore, we provide functional evidence that the oscillation phase shift between Wnt and Notch signaling is critical for PSM segmentation. Our work hence reveals that dynamic signaling, i.e., the relative timing between oscillatory signals, encodes essential information during multicellular development.
Keywords: Notch; Wnt; entrainment; mesoderm segmentation; oscillations; phase shift; presomitic mesoderm; relative timing; signaling dynamics.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures







References
-
- Andrewes W.J.H. The Quest for Longitude. In: Andrewes W.J.H., editor. Proceedings of the Longitude Symposium, Harvard University, Cambridge, Massachusetts, November 4-6, 1993. Harvard University Collection of Historical Scientific Instruments; 1996. p. 437.
-
- Aulehla A., Wehrle C., Brand-Saberi B., Kemler R., Gossler A., Kanzler B., Herrmann B.G. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev. Cell. 2003;4:395–406. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

