Unloosing the Gordian knot of peroxisome formation
- PMID: 29475136
- PMCID: PMC6525147
- DOI: 10.1016/j.ceb.2018.02.002
Unloosing the Gordian knot of peroxisome formation
Abstract
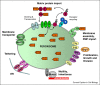
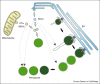
Peroxisome biogenesis is governed by molecular machineries, which are either unique to peroxisomes or are partially shared with mitochondria. As peroxisomes have important protective functions in the cell, modulation of their number is important for human health and disease. Significant progress has been made towards our understanding of the mechanisms of peroxisome formation, revealing a remarkable plasticity of the peroxisome biogenesis pathway. Here we discuss most recent findings with particular focus on peroxisome formation in mammalian cells.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Waterham H.R., Ferdinandusse S., Wanders R.J.A. Human disorders of peroxisome metabolism and biogenesis. Biochim Biophys Acta — Mol Cell Res. 2016;1863:922–933. - PubMed
-
- Delmaghani S., Defourny J., Aghaie A., Beurg M., Dulon D., Thelen N., Perfettini I., Zelles T., Aller M., Meyer A. Hypervulnerability to sound exposure through impaired adaptive proliferation of peroxisomes. Cell. 2015;163:894–906. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

