The minimally effective dose of sucrose for procedural pain relief in neonates: a randomized controlled trial
- PMID: 29475433
- PMCID: PMC5824554
- DOI: 10.1186/s12887-018-1026-x
The minimally effective dose of sucrose for procedural pain relief in neonates: a randomized controlled trial
Abstract
Background: Orally administered sucrose is effective and safe in reducing pain intensity during single, tissue-damaging procedures in neonates, and is commonly recommended in neonatal pain guidelines. However, there is wide variability in sucrose doses examined in research, and more than a 20-fold variation across neonatal care settings. The aim of this study was to determine the minimally effective dose of 24% sucrose for reducing pain in hospitalized neonates undergoing a single skin-breaking heel lance procedure.
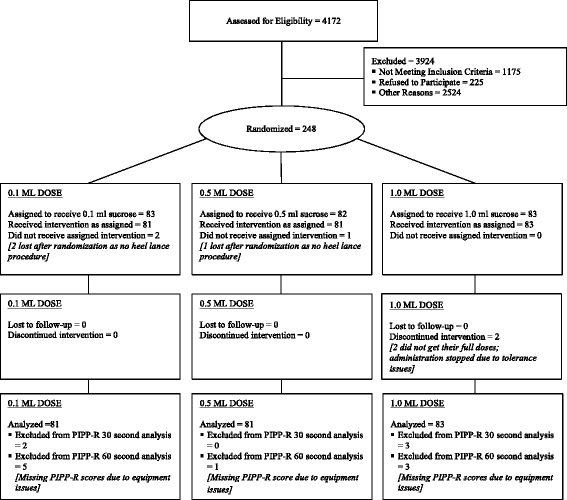
Methods: A total of 245 neonates from 4 Canadian tertiary neonatal intensive care units (NICUs), born between 24 and 42 weeks gestational age (GA), were prospectively randomized to receive one of three doses of 24% sucrose, plus non-nutritive sucking/pacifier, 2 min before a routine heel lance: 0.1 ml (Group 1; n = 81), 0.5 ml (Group 2; n = 81), or 1.0 ml (Group 3; n = 83). The primary outcome was pain intensity measured at 30 and 60 s following the heel lance, using the Premature Infant Pain Profile-Revised (PIPP-R). The secondary outcome was the incidence of adverse events. Analysis of covariance models, adjusting for GA and study site examined between group differences in pain intensity across intervention groups.
Results: There was no difference in mean pain intensity PIPP-R scores between treatment groups at 30 s (P = .97) and 60 s (P = .93); however, pain was not fully eliminated during the heel lance procedure. There were 5 reported adverse events among 5/245 (2.0%) neonates, with no significant differences in the proportion of events by sucrose dose (P = .62). All events resolved spontaneously without medical intervention.
Conclusions: The minimally effective dose of 24% sucrose required to treat pain associated with a single heel lance in neonates was 0.1 ml. Further evaluation regarding the sustained effectiveness of this dose in reducing pain intensity in neonates for repeated painful procedures is warranted.
Trial registration: ClinicalTrials.gov : NCT02134873. Date: May 5, 2014 (retrospectively registered).
Keywords: Adverse event; Analgesia; Heel lance; NICU; Neonates; PIPP-R; Pain; Preterm infants; Sucrose.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Research Ethics Boards at The Hospital for Sick Children (1000038052), Sunnybrook Health Sciences Centre (354–2013), IWK Health Centre (1013855), The Ottawa Hospital (20130327-01H), and Children’s Hospital of Eastern Ontario (13/12E). Informed written consent was obtained from a parent prior to study enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

