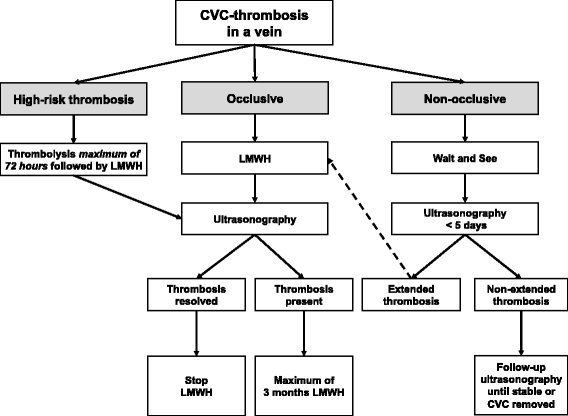
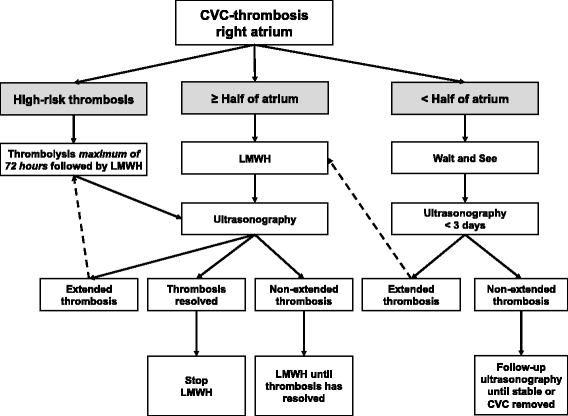
NEOnatal Central-venous Line Observational study on Thrombosis (NEOCLOT): evaluation of a national guideline on management of neonatal catheter-related thrombosis
- PMID: 29475450
- PMCID: PMC5824541
- DOI: 10.1186/s12887-018-1000-7
NEOnatal Central-venous Line Observational study on Thrombosis (NEOCLOT): evaluation of a national guideline on management of neonatal catheter-related thrombosis
Abstract
Background: In critically ill (preterm) neonates, central venous catheters (CVCs) are increasingly used for administration of medication or parenteral nutrition. A serious complication, however, is the development of catheter-related thrombosis (CVC-thrombosis), which may resolve by itself or cause severe complications. Due to lack of evidence, management of neonatal CVC-thrombosis varies among neonatal intensive care units (NICUs). In the Netherlands an expert-based national management guideline has been developed which is implemented in all 10 NICUs in 2014.
Methods: The NEOCLOT study is a multicentre prospective observational cohort study, including 150 preterm and term infants (0-6 months) admitted to one of the 10 NICUs, developing CVC-thrombosis. Patient characteristics, thrombosis characteristics, risk factors, treatment strategies and outcome measures will be collected in a web-based database. Management of CVC-thrombosis will be performed as recommended in the protocol. Violations of the protocol will be noted. Primary outcome measures are a composite efficacy outcome consisting of death due to CVC-thrombosis and recurrent thrombosis, and a safety outcome consisting of the incidence of major bleedings during therapy. Secondary outcomes include individual components of primary efficacy outcome, clinically relevant non-major and minor bleedings and the frequency of risk factors, protocol variations, residual thrombosis and post thrombotic syndrome.
Discussion: The NEOCLOT study will evaluate the efficacy and safety of the new, national, neonatal CVC-thrombosis guideline. Furthermore, risk factors as well as long-term consequences of CVC-thrombosis will be analysed.
Trial registration: Trial registration: Nederlands Trial Register NTR4336 . Registered 24 December 2013.
Keywords: Antithrombotic therapy; Catheter; Neonate; Observational; Thrombosis.
Conflict of interest statement
Ethics approval and consent to participate
The Medical Ethics Review Committee confirmed that official approval of this study was not required as the Medical Research Involving Human Subjects Act did not apply to the NEOCLOT study. (#14.17.0121) Consent to participate was not required.
Consent for publication
Not applicable.
Competing interests
This trial is partly funded by an unrestricted grant of Daiichi Sankyo. Daiichi Sankyo has no role in the design of the study and the collection, analysis and interpretation of data, and in writing the manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
NEOnatal Central-venous Line Observational study on Thrombosis (NEOCLOT): evaluation of a national guideline on management of neonatal catheter-related venous thrombosis.J Thromb Haemost. 2023 Apr;21(4):963-974. doi: 10.1016/j.jtha.2022.11.044. Epub 2022 Dec 22. J Thromb Haemost. 2023. PMID: 36696213
-
Central venous catheterization and thrombosis in newborns: update on diagnosis and management.J Matern Fetal Neonatal Med. 2012 Oct;25 Suppl 4:26-8. doi: 10.3109/14767058.2012.714974. J Matern Fetal Neonatal Med. 2012. Retraction in: J Matern Fetal Neonatal Med. 2014 May;27(8):864. doi: 10.3109/14767058.2013.841387. PMID: 22958007 Retracted. Review.
-
Neonatal central venous catheter thrombosis: diagnosis, management and outcome.Blood Coagul Fibrinolysis. 2014 Mar;25(2):97-106. doi: 10.1097/MBC.0b013e328364f9b0. Blood Coagul Fibrinolysis. 2014. PMID: 24477225 Review.
-
Complications associated with central venous catheters inserted in critically ill neonates.Infect Control Hosp Epidemiol. 1991 Sep;12(9):544-8. doi: 10.1086/646407. Infect Control Hosp Epidemiol. 1991. PMID: 1940277
-
More than a baby step toward improved treatment: Commentary on "NEOnatal Central-venous Line Observational study on Thrombosis (NEOCLOT): evaluation of a national guideline on management of neonatal catheter-related venous thrombosis".J Thromb Haemost. 2023 Apr;21(4):771-772. doi: 10.1016/j.jtha.2022.12.022. J Thromb Haemost. 2023. PMID: 36990518 No abstract available.
Cited by
-
Arterial embolism caused by a peripherally inserted central catheter in a very premature infant: A case report and literature review.World J Clin Cases. 2020 Sep 26;8(18):4259-4265. doi: 10.12998/wjcc.v8.i18.4259. World J Clin Cases. 2020. PMID: 33024787 Free PMC article.
-
Effectiveness and Safety of Nadroparin Therapy in Preterm and Term Neonates with Venous Thromboembolism.J Clin Med. 2021 Apr 2;10(7):1483. doi: 10.3390/jcm10071483. J Clin Med. 2021. PMID: 33918440 Free PMC article.
-
[Peripherally inserted central venous catheter-related thrombosis in a neonate].Zhongguo Dang Dai Er Ke Za Zhi. 2023 Jun 15;25(6):658-662. doi: 10.7499/j.issn.1008-8830.2304051. Zhongguo Dang Dai Er Ke Za Zhi. 2023. PMID: 37382138 Free PMC article. Chinese.
-
Characteristics of Deep Venous Thrombosis in Isolated Lower Extremity Fractures and Unsolved Problems in Guidelines: A Review of Recent Literature.Orthop Surg. 2022 Aug;14(8):1558-1568. doi: 10.1111/os.13306. Epub 2022 May 27. Orthop Surg. 2022. PMID: 35633091 Free PMC article. Review.
-
Neonatal coagulopathies: A review of established and emerging treatments.Exp Biol Med (Maywood). 2021 Jun;246(12):1447-1457. doi: 10.1177/15353702211006046. Epub 2021 Apr 15. Exp Biol Med (Maywood). 2021. PMID: 33858204 Free PMC article. Review.
References
-
- Nowak-Gottl U, Kosch A, Schlegel N. Thromboembolism in newborns, infants and children. Thromb Haemost. 2001;86:464–474. - PubMed
-
- Monagle P, Chan AK, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Gottl U, et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e737S–e801S. doi: 10.1378/chest.11-2308. - DOI - PMC - PubMed
-
- Butler-O'Hara M, Buzzard CJ, Reubens L, McDermott MP, DiGrazio W, D'Angio CT. A randomized trial comparing long-term and short-term use of umbilical venous catheters in premature infants with birth weights of less than 1251 grams. Pediatr. 2006;118:e25–e35. doi: 10.1542/peds.2005-1880. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical