Measuring behavior across scales
- PMID: 29475451
- PMCID: PMC5824583
- DOI: 10.1186/s12915-018-0494-7
Measuring behavior across scales
Abstract
The need for high-throughput, precise, and meaningful methods for measuring behavior has been amplified by our recent successes in measuring and manipulating neural circuitry. The largest challenges associated with moving in this direction, however, are not technical but are instead conceptual: what numbers should one put on the movements an animal is performing (or not performing)? In this review, I will describe how theoretical and data analytical ideas are interfacing with recently-developed computational and experimental methodologies to answer these questions across a variety of contexts, length scales, and time scales. I will attempt to highlight commonalities between approaches and areas where further advances are necessary to place behavior on the same quantitative footing as other scientific fields.
Conflict of interest statement
Competing interests
The author declares no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

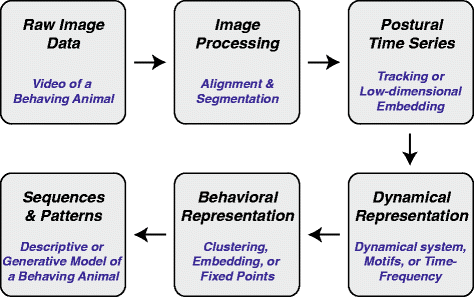
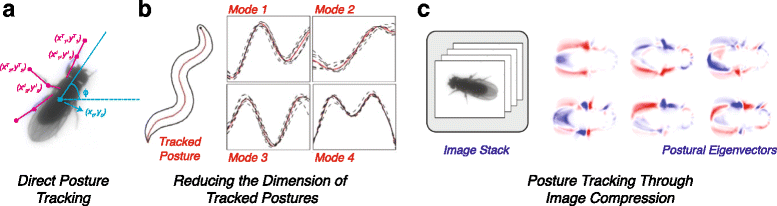
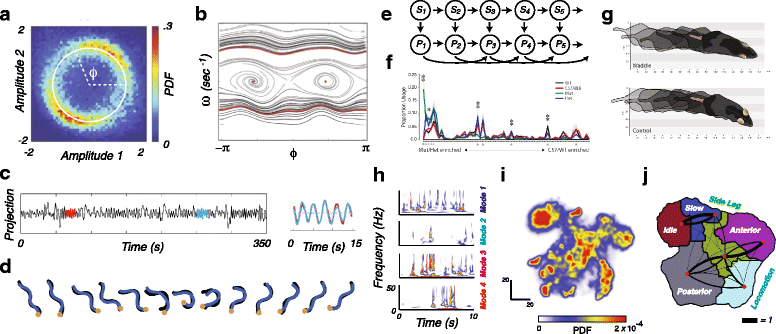
References
-
- Anderson DJ, Perona P. Toward a Science of Computational Ethology. Neuron. 2014;84(1):18–31. - PubMed
-
- Krakauer JW, Ghazanfar AA, Gomez-Marin A, Maciver MA, Poeppel D. Neuroscience Needs Behavior: Correcting a Reductionist Bias. Neuron. 2017;93(3):480–90. - PubMed
-
- Lebedev MA, Nicolelis MAL. Brain-Machine Interfaces: From Basic Science to Neuroprostheses and Neurorehabilitation. Physiol Rev. 2017;97(2):767–837. - PubMed
-
- Anderson PW. More Is Different. Science. 1972;177(4047):393–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

