Burden of Metastatic Castrate Naive Prostate Cancer Patients, to Identify Men More Likely to Benefit from Early Docetaxel: Further Analyses of CHAARTED and GETUG-AFU15 Studies
- PMID: 29475737
- PMCID: PMC6010352
- DOI: 10.1016/j.eururo.2018.02.001
Burden of Metastatic Castrate Naive Prostate Cancer Patients, to Identify Men More Likely to Benefit from Early Docetaxel: Further Analyses of CHAARTED and GETUG-AFU15 Studies
Abstract
Background: Docetaxel (D) at the time of starting androgen deprivation therapy (ADT) for metastatic castrate naive prostate cancer shows a clear survival benefit for patients with high-volume (HV) disease. It is unclear whether patients with low-volume (LV) disease benefit from early D.
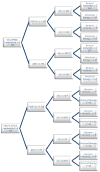
Objective: To define the overall survival (OS) of aggregate data of patient subgroups from the CHAARTED and GETUG-AFU15 studies, defined by metastatic burden (HV and LV) and time of metastasis occurrence (at diagnosis or after prior local treatment [PRLT]).
Design, setting, and participants: Data were accessed from two independent phase III trials of ADT alone or ADT+D-GETUG-AFU15 (N=385) and CHAARTED (N=790), with median follow-ups for survivors of 83.2 and 48.2 mo, respectively. The definition of HV and LV disease was harmonized.
Outcome measurements and statistical analysis: The primary end point was OS.
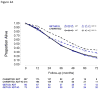
Results and limitations: Meta-analysis results of the aggregate data showed significant heterogeneity in ADT+D versus ADT effect sizes between HV and LV subgroups (p=0.017), and failed to detect heterogeneity in ADT+D versus ADT effect sizes between upfront and PRLT subgroups (p=0.4). Adding D in patients with HV disease has a consistent effect in improving median OS (HV-ADT: 34.4 and 35.1 mo, HV-ADT+D: 51.2 and 39.8 mo in CHAARTED and GETUG-AFU15, respectively; pooled average hazard ratio or HR (95% confidence interval [CI]) 0.68 ([95% CI 0.56; 0.82], p<0.001). Patients with LV disease showed much longer OS, without evidence that D improved OS (LV-ADT: not reached [NR] and 83.4; LV-ADT+D: 63.5 and NR in CHAARTED and GETUG-AFU15, respectively; pooled HR (95% CI) 1.03 (95% CI 0.77; 1.38). Aggregate data showed no evidence of heterogeneity of early D in LV and HV subgroups irrespective of whether patients had PRLT or not. Post hoc subgroup analysis was based on aggregated data from two independent phase III randomized trials.
Conclusions: There was no apparent survival benefit in the CHAARTED and GETUG-AFU15 studies with D for LV. Across both studies, early D showed consistent effect and improved OS in HV patients.
Patient summary: Patients with a higher burden of metastatic prostate cancer starting androgen deprivation therapy (ADT) have a poorer prognosis and are more likely to benefit from early docetaxel. Low-volume patients have longer overall survival with ADT alone, and the toxicity of docetaxel may outweigh its benefits.
Keywords: Androgen deprivation therapy; Chemotherapy; Docetaxel; High volume; Low volume; Metastatic castrate naive prostate cancer; Metastatic prostate cancer; Prostate cancer; Volume disease.
Copyright © 2018 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Figures



Comment in
-
Answering Questions and Questioning Answers: More Evidence To Guide Decision-making About Chemohormonal Therapy in Metastatic Prostate Cancer.Eur Urol. 2018 Jun;73(6):856-858. doi: 10.1016/j.eururo.2018.02.020. Epub 2018 Mar 7. Eur Urol. 2018. PMID: 29525541 No abstract available.
-
[Metastatic, hormone-sensitive prostate cancer: chemo-hormonal therapy for all?].Urologe A. 2018 Aug;57(8):958-959. doi: 10.1007/s00120-018-0696-1. Urologe A. 2018. PMID: 30030598 German. No abstract available.
References
-
- International Agency for Research on Cancer. Prostate cancer: estimated incidence, mortality and prevalence worldwide. 2012 http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
-
- Institut National du Cancer. Les cancers en France/edition 2015. 2016.
-
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. - PubMed
-
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. - PubMed
-
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01 CA004919/CA/NCI NIH HHS/United States
- UG1 CA189829/CA/NCI NIH HHS/United States
- U10 CA027525/CA/NCI NIH HHS/United States
- U10 CA004919/CA/NCI NIH HHS/United States
- P50 CA180995/CA/NCI NIH HHS/United States
- U10 CA013650/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- U10 CA180802/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA049883/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA180801/CA/NCI NIH HHS/United States
- U10 CA107868/CA/NCI NIH HHS/United States
- U10 CA035421/CA/NCI NIH HHS/United States
- U10 CA021076/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- U10 CA180847/CA/NCI NIH HHS/United States
- UG1 CA232760/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- U10 CA180790/CA/NCI NIH HHS/United States
- U10 CA016116/CA/NCI NIH HHS/United States
- UG1 CA233196/CA/NCI NIH HHS/United States
- U10 CA014548/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- U10 CA180833/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- U10 CA180799/CA/NCI NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA180853/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

