Effects of gastroprotectant drugs for the prevention and treatment of peptic ulcer disease and its complications: a meta-analysis of randomised trials
- PMID: 29475806
- PMCID: PMC5842491
- DOI: 10.1016/S2468-1253(18)30037-2
Effects of gastroprotectant drugs for the prevention and treatment of peptic ulcer disease and its complications: a meta-analysis of randomised trials
Abstract
Background: Gastroprotectant drugs are used for the prevention and treatment of peptic ulcer disease and might reduce its associated complications, but reliable estimates of the effects of gastroprotectants in different clinical settings are scarce. We aimed to examine the effects of proton-pump inhibitors (PPIs), prostaglandin analogues, and histamine-2 receptor antagonists (H2RAs) in different clinical circumstances by doing meta-analyses of tabular data from all relevant unconfounded randomised trials of gastroprotectant drugs.
Methods: We searched MEDLINE and Embase from Jan 1, 1950, to Dec 31, 2015, to identify unconfounded, randomised trials of a gastroprotectant drug (defined as a PPI, prostaglandin analogue, or H2RA) versus control, or versus another gastroprotectant. Two independent researchers reviewed the search results and extracted the prespecified outcomes and key characteristics for each trial. We did meta-analyses of the effects of gastroprotectant drugs on ulcer development, bleeding, and mortality overall, according to the class of gastroprotectant, and according to the individual drug within a gastroprotectant class.
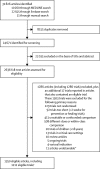
Findings: We identified comparisons of gastroprotectant versus control in 849 trials (142 485 participants): 580 prevention trials (110 626 participants), 233 healing trials (24 033 participants), and 36 trials for the treatment of acute upper gastrointestinal bleeding (7826 participants). Comparisons of one gastroprotectant drug versus another were available in 345 trials (64 905 participants), comprising 160 prevention trials (32 959 participants), 167 healing trials (28 306 participants), and 18 trials for treatment of acute upper gastrointestinal bleeding (3640 participants). The median number of patients in each trial was 78 (IQR 44·0-210·5) and the median duration was 1·4 months (0·9-2·8). In prevention trials, gastroprotectant drugs reduced development of endoscopic ulcers (odds ratio [OR] 0·27, 95% CI 0·25-0·29; p<0·0001), symptomatic ulcers (0·25, 0·22-0·29; p<0·0001), and upper gastrointestinal bleeding (0·40, 0·32-0·50; p<0·0001), but did not significantly reduce mortality (0·85, 0·69-1·04; p=0·11). Larger proportional reductions in upper gastrointestinal bleeding were observed for PPIs than for other gastroprotectant drugs (PPIs 0·21, 99% CI 0·12-0·36; prostaglandin analogues 0·63, 0·35-1·12; H2RAs 0·49, 0·30-0·80; phet=0·0005). Gastroprotectant drugs were effective in preventing bleeding irrespective of the use of non-steroidal anti-inflammatory drugs (phet=0·56). In healing trials, gastroprotectants increased endoscopic ulcer healing (3·49, 95% CI 3·28-3·72; p<0·0001), with PPIs more effective (5·22, 99% CI 4·00-6·80) than prostaglandin analogues (2·27, 1·91-2·70) and H2RAs (3·80, 3·44-4·20; phet<0·0001). In trials among patients with acute bleeding, gastroprotectants reduced further bleeding (OR 0·68, 95% CI 0·60-0·78; p<0·0001), blood transfusion (0·75, 0·65-0·88; p=0·0003), further endoscopic intervention (0·56, 0·45-0·70; p<0·0001), and surgery (0·72, 0·61-0·84; p<0·0001), but did not significantly reduce mortality (OR 0·90, 0·72-1·11; p=0·31). PPIs had larger protective effects than did H2RAs for further bleeding (phet=0·0107) and blood transfusion (phet=0·0130).
Interpretation: Gastroprotectants, in particular PPIs, reduce the risk of peptic ulcer disease and its complications and promote healing of peptic ulcers in a wide range of clinical circumstances. However, this meta-analysis might have overestimated the benefits owing to small study bias.
Funding: UK Medical Research Council and the British Heart Foundation.
Copyright © 2018 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures





Comment in
-
PPIs for prevention and treatment of peptic ulcer.Lancet Gastroenterol Hepatol. 2018 Apr;3(4):214-215. doi: 10.1016/S2468-1253(18)30047-5. Epub 2018 Feb 21. Lancet Gastroenterol Hepatol. 2018. PMID: 29475805 No abstract available.
References
-
- WHO Global health estimates 2014. http://www.who.int/healthinfo/global_burden_disease/estimates (accessed Jan 4, 2018).
-
- Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390:613–624. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

