Dysregulated invertebrate tropomyosin-dectin-1 interaction confers susceptibility to allergic diseases
- PMID: 29475849
- PMCID: PMC5956913
- DOI: 10.1126/sciimmunol.aam9841
Dysregulated invertebrate tropomyosin-dectin-1 interaction confers susceptibility to allergic diseases
Abstract
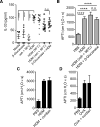
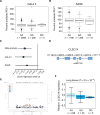
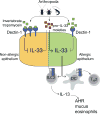
The key factors underlying the development of allergic diseases-the propensity for a minority of individuals to develop dysfunctional responses to harmless environmental molecules-remain undefined. We report a pathway of immune counter-regulation that suppresses the development of aeroallergy and shrimp-induced anaphylaxis. In mice, signaling through epithelially expressed dectin-1 suppresses the development of type 2 immune responses through inhibition of interleukin-33 (IL-33) secretion and the subsequent recruitment of IL-13-producing innate lymphoid cells. Although this homeostatic pathway is functional in respiratory epithelial cells from healthy humans, it is dramatically impaired in epithelial cells from asthmatic and chronic rhinosinusitis patients, resulting in elevated IL-33 production. Moreover, we identify an association between a single-nucleotide polymorphism (SNP) in the dectin-1 gene loci and reduced pulmonary function in two cohorts of asthmatics. This intronic SNP is a predicted eQTL (expression quantitative trait locus) that is associated with reduced dectin-1 expression in human tissue. We identify invertebrate tropomyosin, a ubiquitous arthropod-derived molecule, as an immunobiologically relevant dectin-1 ligand that normally serves to restrain IL-33 release and dampen type 2 immunity in healthy individuals. However, invertebrate tropomyosin presented in the context of impaired dectin-1 function, as observed in allergic individuals, leads to unrestrained IL-33 secretion and skewing of immune responses toward type 2 immunity. Collectively, we uncover a previously unrecognized mechanism of protection against allergy to a conserved recognition element omnipresent in our environment.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures

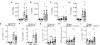
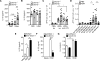


References
-
- Thomas WR, Hales BJ, Smith W-A. Structural biology of allergens. Curr. Allergy Asthma Rep. 2005;5:388–393. - PubMed
-
- Aalberse RC. Structural biology of allergens. J. Allergy Clin. Immunol. 2000;106:228–238. - PubMed
-
- Traidl-Hoffmann C, Jakob T, Behrendt H. Determinants of allergenicity. J. Allergy Clin. Immunol. 2009;123:558–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 ES015794/ES/NIEHS NIH HHS/United States
- R56 AI118791/AI/NIAID NIH HHS/United States
- R01 AI072502/AI/NIAID NIH HHS/United States
- R01 HL078885/HL/NHLBI NIH HHS/United States
- R01 HL135156/HL/NHLBI NIH HHS/United States
- R01 HL104608/HL/NHLBI NIH HHS/United States
- U19 AI070235/AI/NIAID NIH HHS/United States
- R01 HL117004/HL/NHLBI NIH HHS/United States
- T32 ES007141/ES/NIEHS NIH HHS/United States
- R01 AI083315/AI/NIAID NIH HHS/United States
- R01 HL088133/HL/NHLBI NIH HHS/United States
- R01 MD010443/MD/NIMHD NIH HHS/United States
- R01 AI127644/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

