Genus-Wide Assessment of Lignocellulose Utilization in the Extremely Thermophilic Genus Caldicellulosiruptor by Genomic, Pangenomic, and Metagenomic Analyses
- PMID: 29475869
- PMCID: PMC5930323
- DOI: 10.1128/AEM.02694-17
Genus-Wide Assessment of Lignocellulose Utilization in the Extremely Thermophilic Genus Caldicellulosiruptor by Genomic, Pangenomic, and Metagenomic Analyses
Abstract
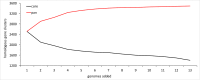
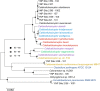
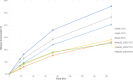
Metagenomic data from Obsidian Pool (Yellowstone National Park, USA) and 13 genome sequences were used to reassess genus-wide biodiversity for the extremely thermophilic Caldicellulosiruptor The updated core genome contains 1,401 ortholog groups (average genome size for 13 species = 2,516 genes). The pangenome, which remains open with a revised total of 3,493 ortholog groups, encodes a variety of multidomain glycoside hydrolases (GHs). These include three cellulases with GH48 domains that are colocated in the glucan degradation locus (GDL) and are specific determinants for microcrystalline cellulose utilization. Three recently sequenced species, Caldicellulosiruptor sp. strain Rt8.B8 (renamed here Caldicellulosiruptor morganii), Thermoanaerobacter cellulolyticus strain NA10 (renamed here Caldicellulosiruptor naganoensis), and Caldicellulosiruptor sp. strain Wai35.B1 (renamed here Caldicellulosiruptor danielii), degraded Avicel and lignocellulose (switchgrass). C. morganii was more efficient than Caldicellulosiruptor bescii in this regard and differed from the other 12 species examined, both based on genome content and organization and in the specific domain features of conserved GHs. Metagenomic analysis of lignocellulose-enriched samples from Obsidian Pool revealed limited new information on genus biodiversity. Enrichments yielded genomic signatures closely related to that of Caldicellulosiruptor obsidiansis, but there was also evidence for other thermophilic fermentative anaerobes (Caldanaerobacter, Fervidobacterium, Caloramator, and Clostridium). One enrichment, containing 89.8% Caldicellulosiruptor and 9.7% Caloramator, had a capacity for switchgrass solubilization comparable to that of C. bescii These results refine the known biodiversity of Caldicellulosiruptor and indicate that microcrystalline cellulose degradation at temperatures above 70°C, based on current information, is limited to certain members of this genus that produce GH48 domain-containing enzymes.IMPORTANCE The genus Caldicellulosiruptor contains the most thermophilic bacteria capable of lignocellulose deconstruction, which are promising candidates for consolidated bioprocessing for the production of biofuels and bio-based chemicals. The focus here is on the extant capability of this genus for plant biomass degradation and the extent to which this can be inferred from the core and pangenomes, based on analysis of 13 species and metagenomic sequence information from environmental samples. Key to microcrystalline hydrolysis is the content of the glucan degradation locus (GDL), a set of genes encoding glycoside hydrolases (GHs), several of which have GH48 and family 3 carbohydrate binding module domains, that function as primary cellulases. Resolving the relationship between the GDL and lignocellulose degradation will inform efforts to identify more prolific members of the genus and to develop metabolic engineering strategies to improve this characteristic.
Keywords: Caldicellulosiruptor; extreme thermophile; pangenome.
Copyright © 2018 American Society for Microbiology.
Figures





References
-
- van de Werken HJG, Verhaart MRA, VanFossen AL, Willquist K, Lewis DL, Nichols JD, Goorissen HP, Mongodin EF, Nelson KE, van Niel EWJ, Stams AJM, Ward DE, de Vos WM, van der Oost J, Kelly RM, Kengen SWM. 2008. Hydrogenomics of the extremely thermophilic bacterium Caldicellulosiruptor saccharolyticus. Appl Environ Microbiol 74:6720–6729. doi: 10.1128/AEM.00968-08. - DOI - PMC - PubMed
-
- Blumer-Schuette SE, Giannone RJ, Zurawski JV, Ozdemir I, Ma Q, Yin Y, Xu Y, Kataeva I, Poole FL, Adams MWW, Hamilton-Brehm SD, Elkins JG, Larimer FW, Land ML, Hauser LJ, Cottingham RW, Hettich RL, Kelly RM. 2012. Caldicellulosiruptor core and pangenomes reveal determinants for noncellulosomal thermophilic deconstruction of plant biomass. J Bacteriol 194:4015–4028. doi: 10.1128/JB.00266-12. - DOI - PMC - PubMed
-
- Brunecky R, Alahuhta M, Xu Q, Donohoe BS, Crowley MF, Kataeva IA, Yang S-J, Resch MG, Adams MWW, Lunin VV, Himmel ME, Bomble YJ. 2013. Revealing nature's cellulase diversity: the digestion mechanism of Caldicellulosiruptor bescii CelA. Science 342:1513–1516. doi: 10.1126/science.1244273. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

