Antiretroviral Drug Metabolism in Humanized PXR-CAR-CYP3A-NOG Mice
- PMID: 29476044
- PMCID: PMC5878674
- DOI: 10.1124/jpet.117.247288
Antiretroviral Drug Metabolism in Humanized PXR-CAR-CYP3A-NOG Mice
Abstract
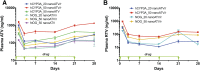
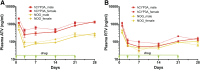
Antiretroviral drug (ARV) metabolism is linked largely to hepatic cytochrome P450 activity. One ARV drug class known to be metabolized by intestinal and hepatic CYP3A are the protease inhibitors (PIs). Plasma drug concentrations are boosted by CYP3A inhibitors such as cobisistat and ritonavir (RTV). Studies of such drug-drug interactions are limited since the enzyme pathways are human specific. While immune-deficient mice reconstituted with human cells are an excellent model to study ARVs during human immunodeficiency virus type 1 (HIV-1) infection, they cannot reflect human drug metabolism. Thus, we created a mouse strain with the human pregnane X receptor, constitutive androstane receptor, and CYP3A4/7 genes on a NOD.Cg-Prkdcscid Il2rgtm1Sug /JicTac background (hCYP3A-NOG) and used them to evaluate the impact of human CYP3A metabolism on ARV pharmacokinetics. In proof-of-concept studies we used nanoformulated atazanavir (nanoATV) with or without RTV. NOG and hCYP3A-NOG mice were treated weekly with 50 mg/kg nanoATV alone or boosted with nanoformulated ritonavir (nanoATV/r). Plasma was collected weekly and liver was collected at 28 days post-treatment. Plasma and liver atazanavir (ATV) concentrations in nanoATV/r-treated hCYP3A-NOG mice were 2- to 4-fold higher than in replicate NOG mice. RTV enhanced plasma and liver ATV concentrations 3-fold in hCYP3A-NOG mice and 1.7-fold in NOG mice. The results indicate that human CYP3A-mediated drug metabolism is reduced compared with mouse and that RTV differentially affects human gene activity. These differences can affect responses to PIs in humanized mouse models of HIV-1 infection. Importantly, hCYP3A-NOG mice reconstituted with human immune cells can be used for bench-to-bedside translation.
Copyright © 2018 by The Author(s).
Figures
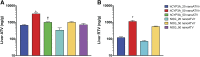

References
-
- Arya V, Robertson SM, Struble KA, Murray JS. (2012) Scientific considerations for pharmacoenhancers in antiretroviral therapy. J Clin Pharmacol 52:1128–1133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

