Membrane cholesterol mediates the cellular effects of monolayer graphene substrates
- PMID: 29476054
- PMCID: PMC5824811
- DOI: 10.1038/s41467-018-03185-0
Membrane cholesterol mediates the cellular effects of monolayer graphene substrates
Abstract
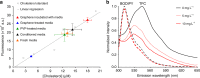
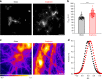
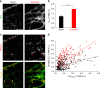
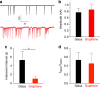
Graphene possesses extraordinary properties that promise great potential in biomedicine. However, fully leveraging these properties requires close contact with the cell surface, raising the concern of unexpected biological consequences. Computational models have demonstrated that graphene preferentially interacts with cholesterol, a multifunctional lipid unique to eukaryotic membranes. Here we demonstrate an interaction between graphene and cholesterol. We find that graphene increases cell membrane cholesterol and potentiates neurotransmission, which is mediated by increases in the number, release probability, and recycling rate of synaptic vesicles. In fibroblasts grown on graphene, we also find an increase in cholesterol, which promotes the activation of P2Y receptors, a family of receptor regulated by cholesterol. In both cases, direct manipulation of cholesterol levels elucidates that a graphene-induced cholesterol increase underlies the observed potentiation of each cell signaling pathway. These findings identify cholesterol as a mediator of graphene's cellular effects, providing insight into the biological impact of graphene.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Similar articles
-
Cholesterol Extraction from Cell Membrane by Graphene Nanosheets: A Computational Study.J Phys Chem B. 2016 Feb 11;120(5):957-64. doi: 10.1021/acs.jpcb.5b10330. Epub 2016 Feb 3. J Phys Chem B. 2016. PMID: 26812232
-
An Increase in Membrane Cholesterol by Graphene Oxide Disrupts Calcium Homeostasis in Primary Astrocytes.Small. 2019 Apr;15(15):e1900147. doi: 10.1002/smll.201900147. Epub 2019 Mar 20. Small. 2019. PMID: 30891923
-
Dewetting transition assisted clearance of (NFGAILS) amyloid fibrils from cell membranes by graphene.J Chem Phys. 2014 Dec 14;141(22):22D520. doi: 10.1063/1.4901113. J Chem Phys. 2014. PMID: 25494791
-
Cytotoxicity of graphene: recent advances and future perspective.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014 Sep-Oct;6(5):452-74. doi: 10.1002/wnan.1277. Epub 2014 Jun 23. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014. PMID: 24957946 Review.
-
Interactions of graphene with mammalian cells: Molecular mechanisms and biomedical insights.Adv Drug Deliv Rev. 2016 Oct 1;105(Pt B):145-162. doi: 10.1016/j.addr.2016.08.009. Epub 2016 Aug 26. Adv Drug Deliv Rev. 2016. PMID: 27569910 Review.
Cited by
-
Impact of Graphene on the Efficacy of Neuron Culture Substrates.Adv Healthc Mater. 2018 Jul;7(14):e1701290. doi: 10.1002/adhm.201701290. Epub 2018 Jun 25. Adv Healthc Mater. 2018. PMID: 29943431 Free PMC article.
-
Cholesterols Induced Distinctive Entry of the Graphene Nanosheet into the Cell Membrane.ACS Omega. 2024 Feb 19;9(8):9216-9225. doi: 10.1021/acsomega.3c08236. eCollection 2024 Feb 27. ACS Omega. 2024. PMID: 38434853 Free PMC article.
-
Graphene-Based Nanomaterials: From Production to Integration With Modern Tools in Neuroscience.Front Syst Neurosci. 2019 Jul 16;13:26. doi: 10.3389/fnsys.2019.00026. eCollection 2019. Front Syst Neurosci. 2019. PMID: 31379522 Free PMC article.
-
Is Graphene Shortening the Path toward Spinal Cord Regeneration?ACS Nano. 2022 Sep 27;16(9):13430-13467. doi: 10.1021/acsnano.2c04756. Epub 2022 Aug 24. ACS Nano. 2022. PMID: 36000717 Free PMC article. Review.
-
Interactions Between 2D Materials and Living Matter: A Review on Graphene and Hexagonal Boron Nitride Coatings.Front Bioeng Biotechnol. 2021 Jan 27;9:612669. doi: 10.3389/fbioe.2021.612669. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33585432 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

