An all-trans-retinal-binding opsin peropsin as a potential dark-active and light-inactivated G protein-coupled receptor
- PMID: 29476064
- PMCID: PMC5824942
- DOI: 10.1038/s41598-018-21946-1
An all-trans-retinal-binding opsin peropsin as a potential dark-active and light-inactivated G protein-coupled receptor
Abstract
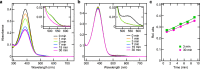
Peropsin or retinal pigment epithelium-derived rhodopsin homolog, found in many animals, belongs to the opsin family. Most opsins bind to 11-cis-retinal as a chromophore and act as light-activated G protein-coupled receptors. Some peropsins, however, bind all-trans-retinal and isomerise it into 11-cis form by light, and peropsin has been suggested to supply other visual opsins with 11-cis-retinal. Additionally, peropsin has some amino acid sequence motifs that are highly conserved among G protein-coupled opsins. Here, using chimeric mutant peropsins, we found that peropsin potentially generates an "active form" that drives G-protein signalling in the dark by binding to all-trans-retinal and that the active form photo-converts to an inactive form containing 11-cis-retinal. Comparative spectroscopic analysis demonstrated that spider peropsin exhibited catalytic efficiency for retinal photoisomerisation that was much lower than a retinal photoisomerase, squid retinochrome. The chimeric peropsins, constructed by replacing the third intracellular loop region with that of Gs- or Gi-coupled opsin, were active and drove Gs- or Gi-mediated signalling in the dark, respectively, and were inactivated upon illumination in mammalian cultured cells. These results suggest that peropsin acts as a dark-active, light-inactivated G protein-coupled receptor and is useful as a novel optogenetic tool.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Peropsin modulates transit of vitamin A from retina to retinal pigment epithelium.J Biol Chem. 2017 Dec 29;292(52):21407-21416. doi: 10.1074/jbc.M117.812701. Epub 2017 Nov 6. J Biol Chem. 2017. PMID: 29109151 Free PMC article.
-
Identification and characterization of a protostome homologue of peropsin from a jumping spider.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010 Jan;196(1):51-9. doi: 10.1007/s00359-009-0493-9. Epub 2009 Dec 4. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010. PMID: 19960196
-
Amphioxus homologs of Go-coupled rhodopsin and peropsin having 11-cis- and all-trans-retinals as their chromophores.FEBS Lett. 2002 Nov 20;531(3):525-8. doi: 10.1016/s0014-5793(02)03616-5. FEBS Lett. 2002. PMID: 12435605
-
Optogenetic Potentials of Diverse Animal Opsins: Parapinopsin, Peropsin, LWS Bistable Opsin.Adv Exp Med Biol. 2021;1293:141-151. doi: 10.1007/978-981-15-8763-4_8. Adv Exp Med Biol. 2021. PMID: 33398811 Review.
-
Diversity of animal opsin-based pigments and their optogenetic potential.Biochim Biophys Acta. 2014 May;1837(5):710-6. doi: 10.1016/j.bbabio.2013.09.003. Epub 2013 Sep 13. Biochim Biophys Acta. 2014. PMID: 24041647 Review.
Cited by
-
Optogenetic Modulation of Ion Channels by Photoreceptive Proteins.Adv Exp Med Biol. 2021;1293:73-88. doi: 10.1007/978-981-15-8763-4_5. Adv Exp Med Biol. 2021. PMID: 33398808 Review.
-
Rhodopsins: An Excitingly Versatile Protein Species for Research, Development and Creative Engineering.Front Chem. 2022 Jun 22;10:879609. doi: 10.3389/fchem.2022.879609. eCollection 2022. Front Chem. 2022. PMID: 35815212 Free PMC article. Review.
-
The Gluopsins: Opsins without the Retinal Binding Lysine.Cells. 2022 Aug 6;11(15):2441. doi: 10.3390/cells11152441. Cells. 2022. PMID: 35954284 Free PMC article.
-
A variety of photoreceptors and the frontiers of optogenetics.Biophys Physicobiol. 2022 Feb 9;19:1-3. doi: 10.2142/biophysico.bppb-v19.0004. eCollection 2022. Biophys Physicobiol. 2022. PMID: 35532380 Free PMC article. No abstract available.
-
Long-wave opsin involved in body color plastic development in Nilaparvata lugens.BMC Genomics. 2023 Jun 26;24(1):353. doi: 10.1186/s12864-023-09470-7. BMC Genomics. 2023. PMID: 37365539 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

