Vertical Transmission of the Zika Virus Causes Neurological Disorders in Mouse Offspring
- PMID: 29476066
- PMCID: PMC5824946
- DOI: 10.1038/s41598-018-21894-w
Vertical Transmission of the Zika Virus Causes Neurological Disorders in Mouse Offspring
Abstract
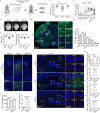
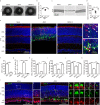
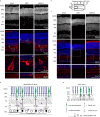
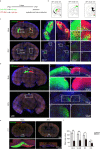
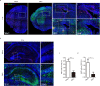
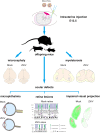
The association between Zika virus (ZIKV) infection and congenital malformations such as microcephaly in infants is a public health emergency. Although various in vivo and in vitro models are used for ZIKV research, few animal models are available for resolving the effects of maternal ZIKV infection on neonatal development. Here, we established an immunocompetent mouse model via intrauterine inoculation. Our results confirmed that ZIKV, but not dengue virus, infection caused spontaneous abortions, brain malformations, ocular abnormalities, spinal cord defects and paralysis in mouse offspring. Aside from microcephaly and hippocampal dysplasia, eye abnormalities, including microphthalmia, thinner optic nerves, damaged retinae, and deficient visual projection, were also observed following ZIKV infection. Moreover, ZIKV-infected offspring showed a loss of alpha motor neurons in the spinal cord and cerebellar malformation, which may cause paralysis. ZIKV also impaired adult neurogenesis in neonatal mice. Due to its intact immunity, our rodent model can be used to systematically evaluate the impact of ZIKV on embryonic and neonatal development and to explore potential therapies.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Progressive lesions of central nervous system in microcephalic fetuses with suspected congenital Zika virus syndrome.Ultrasound Obstet Gynecol. 2017 Dec;50(6):717-722. doi: 10.1002/uog.17303. Epub 2017 Nov 8. Ultrasound Obstet Gynecol. 2017. PMID: 27644020
-
Zika Virus Infection in Pregnancy, Microcephaly, and Maternal and Fetal Health: What We Think, What We Know, and What We Think We Know.Arch Pathol Lab Med. 2017 Jan;141(1):26-32. doi: 10.5858/arpa.2016-0382-RA. Epub 2016 Sep 16. Arch Pathol Lab Med. 2017. PMID: 27636525 Review.
-
Congenital Zika Virus Infection: Beyond Neonatal Microcephaly.JAMA Neurol. 2016 Dec 1;73(12):1407-1416. doi: 10.1001/jamaneurol.2016.3720. JAMA Neurol. 2016. PMID: 27695855
-
Zika virus infection at mid-gestation results in fetal cerebral cortical injury and fetal death in the olive baboon.PLoS Pathog. 2019 Jan 18;15(1):e1007507. doi: 10.1371/journal.ppat.1007507. eCollection 2019 Jan. PLoS Pathog. 2019. PMID: 30657788 Free PMC article.
-
Insights into the molecular roles of Zika virus in human reproductive complications and congenital neuropathologies.Pathology. 2017 Dec;49(7):707-714. doi: 10.1016/j.pathol.2017.07.008. Epub 2017 Oct 7. Pathology. 2017. PMID: 29017720 Review.
Cited by
-
Animal models of congenital zika syndrome provide mechanistic insight into viral pathogenesis during pregnancy.PLoS Negl Trop Dis. 2020 Oct 22;14(10):e0008707. doi: 10.1371/journal.pntd.0008707. eCollection 2020 Oct. PLoS Negl Trop Dis. 2020. PMID: 33091001 Free PMC article. Review.
-
Zika virus induced microcephaly and aberrant hematopoietic cell differentiation modeled in novel neonatal humanized mice.Front Immunol. 2023 Feb 7;14:1060959. doi: 10.3389/fimmu.2023.1060959. eCollection 2023. Front Immunol. 2023. PMID: 36825016 Free PMC article.
-
Reversing neural circuit and behavior deficit in mice exposed to maternal inflammation by Zika virus.EMBO Rep. 2021 Aug 4;22(8):e51978. doi: 10.15252/embr.202051978. Epub 2021 Jul 7. EMBO Rep. 2021. PMID: 34232545 Free PMC article.
-
Zika virus induces neuronal and vascular degeneration in developing mouse retina.Acta Neuropathol Commun. 2021 May 25;9(1):97. doi: 10.1186/s40478-021-01195-6. Acta Neuropathol Commun. 2021. PMID: 34034828 Free PMC article.
-
Knowledge, Awareness, and Perception of Community Pharmacists to Zika Virus Infection in Klang Valley, Malaysia.Health Serv Insights. 2020 May 28;13:1178632920921425. doi: 10.1177/1178632920921425. eCollection 2020. Health Serv Insights. 2020. PMID: 32528223 Free PMC article.
References
-
- Besnard, M., Lastere, S., Teissier, A., Cao-Lormeau, V. & Musso, D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill19 (2014). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

