A genetic variant in SLC30A2 causes breast dysfunction during lactation by inducing ER stress, oxidative stress and epithelial barrier defects
- PMID: 29476070
- PMCID: PMC5824919
- DOI: 10.1038/s41598-018-21505-8
A genetic variant in SLC30A2 causes breast dysfunction during lactation by inducing ER stress, oxidative stress and epithelial barrier defects
Abstract
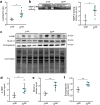
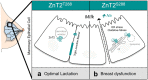
SLC30A2 encodes a zinc (Zn) transporter (ZnT2) that imports Zn into vesicles in highly-specialized secretory cells. Numerous mutations and non-synonymous variants in ZnT2 have been reported in humans and in breastfeeding women; ZnT2 variants are associated with abnormally low milk Zn levels and can lead to severe infantile Zn deficiency. However, ZnT2-null mice have profound defects in mammary epithelial cell (MEC) polarity and vesicle secretion, indicating that normal ZnT2 function is critical for MEC function. Here we report that women who harbor a common ZnT2 variant (T288S) present with elevated levels of several oxidative and endoplasmic reticulum (ER) stress markers in their breast milk. Functional studies in vitro suggest that substitution of threonine for serine at amino acid 288 leads to hyperphosphorylation retaining ZnT2 in the ER and lysosomes, increasing ER and lysosomal Zn accumulation, ER stress, the generation of reactive oxygen species, and STAT3 activation. These changes were associated with decreased abundance of zona occludens-1 and increased tight junction permeability. This study confirms that ZnT2 is important for normal breast function in women during lactation, and suggests that women who harbor defective variants in ZnT2 may be at-risk for poor lactation performance.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Exome Sequencing of SLC30A2 Identifies Novel Loss- and Gain-of-Function Variants Associated with Breast Cell Dysfunction.J Mammary Gland Biol Neoplasia. 2015 Dec;20(3-4):159-72. doi: 10.1007/s10911-015-9338-z. Epub 2015 Aug 21. J Mammary Gland Biol Neoplasia. 2015. PMID: 26293594
-
A common genetic variant in zinc transporter ZnT2 (Thr288Ser) is present in women with low milk volume and alters lysosome function and cell energetics.Am J Physiol Cell Physiol. 2020 Jun 1;318(6):C1166-C1177. doi: 10.1152/ajpcell.00383.2019. Epub 2020 Apr 22. Am J Physiol Cell Physiol. 2020. PMID: 32320289
-
Milk-derived miRNA profiles elucidate molecular pathways that underlie breast dysfunction in women with common genetic variants in SLC30A2.Sci Rep. 2019 Sep 3;9(1):12686. doi: 10.1038/s41598-019-48987-4. Sci Rep. 2019. PMID: 31481661 Free PMC article.
-
The role of the zinc transporter SLC30A2/ZnT2 in transient neonatal zinc deficiency.Metallomics. 2017 Oct 18;9(10):1352-1366. doi: 10.1039/c7mt00162b. Metallomics. 2017. PMID: 28665435 Review.
-
Physiology of milk secretion.Best Pract Res Clin Endocrinol Metab. 2017 Aug;31(4):367-384. doi: 10.1016/j.beem.2017.10.008. Epub 2017 Oct 31. Best Pract Res Clin Endocrinol Metab. 2017. PMID: 29221566 Review.
Cited by
-
Mutation in FBXO32 causes dilated cardiomyopathy through up-regulation of ER-stress mediated apoptosis.Commun Biol. 2021 Jul 16;4(1):884. doi: 10.1038/s42003-021-02391-9. Commun Biol. 2021. PMID: 34272480 Free PMC article.
-
Emerging Perspectives in Zinc Transporter Research in Prostate Cancer: An Updated Review.Nutrients. 2024 Jun 26;16(13):2026. doi: 10.3390/nu16132026. Nutrients. 2024. PMID: 38999774 Free PMC article. Review.
-
Maintenance of Intestinal Epithelial Homeostasis by Zinc Transporters.Dig Dis Sci. 2019 Sep;64(9):2404-2415. doi: 10.1007/s10620-019-05561-2. Epub 2019 Mar 4. Dig Dis Sci. 2019. PMID: 30830525 Review.
-
ER stress in obesity pathogenesis and management.Trends Pharmacol Sci. 2022 Feb;43(2):97-109. doi: 10.1016/j.tips.2021.11.011. Epub 2021 Dec 8. Trends Pharmacol Sci. 2022. PMID: 34893351 Free PMC article. Review.
-
Transient Symptomatic Zinc Deficiency in a Breastfed Infant Associated with Low Zinc Levels in Maternal Serum and Breast Milk Improving after Zinc Supplementation: An Uncommon Phenotype?Indian Dermatol Online J. 2020 Jan 24;11(4):623-626. doi: 10.4103/idoj.IDOJ_386_19. eCollection 2020 Jul-Aug. Indian Dermatol Online J. 2020. PMID: 32832457 Free PMC article.
References
-
- Alam, S., Hennigar, S. R., Gallagher, C., Soybel, D. I. & Kelleher, S. L. Exome Sequencing of SLC30A2 Identifies Novel Loss- and Gain-of-Function Variants Associated with Breast Cell Dysfunction. J Mammary Gland Biol Neoplasia, 10.1007/s10911-015-9338-z (2015). - PubMed
-
- Miletta MC, et al. Transient Neonatal Zinc Deficiency Caused by a Heterozygous G87R Mutation in the Zinc Transporter ZnT-2 (SLC30A2) Gene in the Mother Highlighting the Importance of Zn (2+) for Normal Growth and Development. Int. J. Endocrinol. 2013;2013:259189. doi: 10.1155/2013/259189. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

