STAT3 regulates cytotoxicity of human CD57+ CD4+ T cells in blood and lymphoid follicles
- PMID: 29476109
- PMCID: PMC5824848
- DOI: 10.1038/s41598-018-21389-8
STAT3 regulates cytotoxicity of human CD57+ CD4+ T cells in blood and lymphoid follicles
Abstract
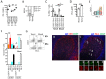
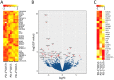
A subset of human follicular helper T cells (TFH) cells expresses CD57 for which no distinct function has been identified. We show that CD57+ TFH cells are universally PD-1hi, but compared to their CD57- PD-1hi counterparts, express little IL-21 or IL-10 among others. Instead, CD57 expression on TFH cells marks cytotoxicity transcriptional signatures that translate into only a weak cytotoxic phenotype. Similarly, circulating PD-1+ CD57+ CD4+ T cells make less cytokine than their CD57- PD-1+ counterparts, but have a prominent cytotoxic phenotype. By analysis of responses to STAT3-dependent cytokines and cells from patients with gain- or loss-of-function STAT3 mutations, we show that CD4+ T cell cytotoxicity is STAT3-dependent. TFH formation also requires STAT3, but paradoxically, once formed, PD-1hi cells become unresponsive to STAT3. These findings suggest that changes in blood and germinal center cytotoxicity might be affected by changes in STAT3 signaling, or modulation of PD-1 by therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

