Allopurinol partially prevents disuse muscle atrophy in mice and humans
- PMID: 29476130
- PMCID: PMC5824846
- DOI: 10.1038/s41598-018-21552-1
Allopurinol partially prevents disuse muscle atrophy in mice and humans
Abstract
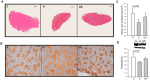
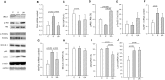
Disuse muscle wasting will likely affect everyone in his or her lifetime in response to pathologies such as joint immobilization, inactivity or bed rest. There are no good therapies to treat it. We previously found that allopurinol, a drug widely used to treat gout, protects muscle damage after exhaustive exercise and results in functional gains in old individuals. Thus, we decided to test its effect in the prevention of soleus muscle atrophy after two weeks of hindlimb unloading in mice, and lower leg immobilization following ankle sprain in humans (EudraCT: 2011-003541-17). Our results show that allopurinol partially protects against muscle atrophy in both mice and humans. The protective effect of allopurinol is similar to that of resistance exercise which is the best-known way to prevent muscle mass loss in disuse human models. We report that allopurinol protects against the loss of muscle mass by inhibiting the expression of ubiquitin ligases. Our results suggest that the ubiquitin-proteasome pathway is an appropriate therapeutic target to inhibit muscle wasting and emphasizes the role of allopurinol as a non-hormonal intervention to treat disuse muscle atrophy.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

