Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation
- PMID: 29476152
- PMCID: PMC5826585
- DOI: 10.1038/s41556-018-0045-z
Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation
Erratum in
-
Author Correction: Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation.Nat Cell Biol. 2018 Sep;20(9):1098. doi: 10.1038/s41556-018-0102-7. Nat Cell Biol. 2018. PMID: 29880862
-
Publisher Correction: Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation.Nat Cell Biol. 2020 Oct;22(10):1288. doi: 10.1038/s41556-020-00580-y. Nat Cell Biol. 2020. PMID: 32855523
Abstract
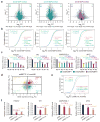
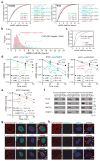
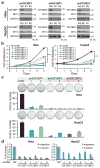
N6-methyladenosine (m6A) is the most prevalent modification in eukaryotic messenger RNAs (mRNAs) and is interpreted by its readers, such as YTH domain-containing proteins, to regulate mRNA fate. Here, we report the insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs; including IGF2BP1/2/3) as a distinct family of m6A readers that target thousands of mRNA transcripts through recognizing the consensus GG(m6A)C sequence. In contrast to the mRNA-decay-promoting function of YTH domain-containing family protein 2, IGF2BPs promote the stability and storage of their target mRNAs (for example, MYC) in an m6A-dependent manner under normal and stress conditions and therefore affect gene expression output. Moreover, the K homology domains of IGF2BPs are required for their recognition of m6A and are critical for their oncogenic functions. Thus, our work reveals a different facet of the m6A-reading process that promotes mRNA stability and translation, and highlights the functional importance of IGF2BPs as m6A readers in post-transcriptional gene regulation and cancer biology.
Conflict of interest statement
C.He is a scientific founder of Accent Therapeutics, Inc.
Figures




Comment in
-
An additional class of m6A readers.Nat Cell Biol. 2018 Mar;20(3):230-232. doi: 10.1038/s41556-018-0046-y. Nat Cell Biol. 2018. PMID: 29476150 Free PMC article.
References
-
- Dominissini D, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RM1 HG008935/HG/NHGRI NIH HHS/United States
- R01 CA236399/CA/NCI NIH HHS/United States
- R01 CA182528/CA/NCI NIH HHS/United States
- R01 GM088599/GM/NIGMS NIH HHS/United States
- R01 CA214965/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- S10 RR027015/RR/NCRR NIH HHS/United States
- R01 CA163493/CA/NCI NIH HHS/United States
- R01 CA178454/CA/NCI NIH HHS/United States
- R01 CA211614/CA/NCI NIH HHS/United States
- R01 CA211066/CA/NCI NIH HHS/United States
- R01 GM113194/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

