Taste and smell perception and quality of life during and after systemic therapy for breast cancer
- PMID: 29476290
- PMCID: PMC5993854
- DOI: 10.1007/s10549-018-4720-3
Taste and smell perception and quality of life during and after systemic therapy for breast cancer
Abstract
Purpose: The purpose of the study was to assess self-reported taste and smell perception after chemotherapy in breast cancer patients compared with women without cancer, and to assess whether taste and smell perception is associated with quality of life after the end of chemotherapy.
Methods: We included 135 newly diagnosed breast cancer patients who completed chemotherapy and 114 women without cancer. Questionnaires on taste, smell, and quality of life were completed shortly after and 6 months after chemotherapy (patients) or at two moments with 6 months' time window in between (comparisons).
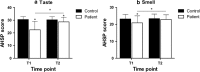
Results: Self-reported taste and smell perception were significantly lower in patients shortly after chemotherapy compared to the comparison group. Most patients recovered 6 months after chemotherapy, although patients who were still receiving trastuzumab then reported a lower taste and smell perception compared to patients who were not. A lower self-reported taste and smell were statistically significantly associated with a worse quality of life, social, emotional, and role functioning shortly after chemotherapy. Six months after chemotherapy, taste and smell were statistically significantly associated with quality of life, social and role functioning, but only in patients receiving trastuzumab.
Conclusions: Most taste and smell alterations recovered within 6 months after the end of chemotherapy for breast cancer, but not for patients receiving trastuzumab. These results highlight the importance of monitoring taste and smell alterations during and after treatment with chemotherapy and trastuzumab, as they may impact quality of life.
Keywords: Breast cancer; Chemotherapy; Dysgeusia; Herceptin; Quality of life; Smell; Taste; Taste loss; Trastuzumab.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in the study described in this manuscript were in accordance with the ethical standards of the institutional and/or national research committee.
Figures

References
-
- de Vries YC, Helmich E, Karsten MDA, Boesveldt S, Winkels RM, van Laarhoven HWM. The impact of chemosensory and food-related changes in patients with advanced oesophagogastric cancer treated with capecitabine and oxaliplatin: a qualitative study. Support Care Cancer. 2016;24(7):3119–3126. - PMC - PubMed
-
- Gamper EM, Giesinger JM, Oberguggenberger A, Kemmler G, Wintner LM, Gattringer K, Sperner-Unterweger B, Holzner B, Zabernigg A. Taste alterations in breast and gynaecological cancer patients receiving chemotherapy: prevalence, course of severity, and quality of life correlates. Acta Oncol. 2012;51(4):490–496. doi: 10.3109/0284186X.2011.633554. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

