Differential stem cell aging kinetics in Hutchinson-Gilford progeria syndrome and Werner syndrome
- PMID: 29476423
- PMCID: PMC5876188
- DOI: 10.1007/s13238-018-0517-8
Differential stem cell aging kinetics in Hutchinson-Gilford progeria syndrome and Werner syndrome
Abstract
Hutchinson-Gilford progeria syndrome (HGPS) and Werner syndrome (WS) are two of the best characterized human progeroid syndromes. HGPS is caused by a point mutation in lamin A (LMNA) gene, resulting in the production of a truncated protein product-progerin. WS is caused by mutations in WRN gene, encoding a loss-of-function RecQ DNA helicase. Here, by gene editing we created isogenic human embryonic stem cells (ESCs) with heterozygous (G608G/+) or homozygous (G608G/G608G) LMNA mutation and biallelic WRN knockout, for modeling HGPS and WS pathogenesis, respectively. While ESCs and endothelial cells (ECs) did not present any features of premature senescence, HGPS- and WS-mesenchymal stem cells (MSCs) showed aging-associated phenotypes with different kinetics. WS-MSCs had early-onset mild premature aging phenotypes while HGPS-MSCs exhibited late-onset acute premature aging characterisitcs. Taken together, our study compares and contrasts the distinct pathologies underpinning the two premature aging disorders, and provides reliable stem-cell based models to identify new therapeutic strategies for pathological and physiological aging.
Keywords: HGPS; WRN; Werner syndrome; aging; lamin; stem cell.
Figures
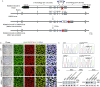

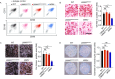
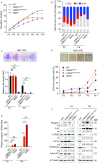



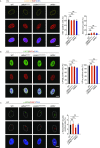


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

