Prospective Evaluation of Two iStent® Trabecular Stents, One iStent Supra® Suprachoroidal Stent, and Postoperative Prostaglandin in Refractory Glaucoma: 4-year Outcomes
- PMID: 29476443
- PMCID: PMC5859115
- DOI: 10.1007/s12325-018-0666-4
Prospective Evaluation of Two iStent® Trabecular Stents, One iStent Supra® Suprachoroidal Stent, and Postoperative Prostaglandin in Refractory Glaucoma: 4-year Outcomes
Abstract
Introduction: This study evaluates long-term outcomes of two trabecular micro-bypass stents, one suprachoroidal stent, and postoperative prostaglandin in eyes with refractory open angle glaucoma (OAG).
Methods: Prospective ongoing 5-year study of 80 eligible subjects (70 with 4-year follow-up) with OAG and IOP ≥ 18 mmHg after prior trabeculectomy and while taking 1-3 glaucoma medications. Subjects received two iStent® trabecular micro-bypass stents, one iStent Supra® suprachoroidal stent, and postoperative travoprost. Postoperative IOP was measured with medication and annually following medication washouts. Performance was measured by the proportion of eyes with ≥ 20% IOP reduction on one medication (the protocol-specified prostaglandin) versus preoperative medicated IOP (primary outcome); and the proportion of eyes with postoperative IOP ≤ 15 and ≤ 18 mmHg on one medication (secondary outcome). Additional clinical and safety data included medications, visual field, pachymetry, gonioscopy, adverse events, visual acuity, and slit-lamp and fundus examinations.
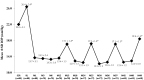
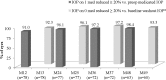
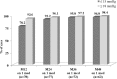
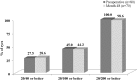
Results: Preoperatively, mean medicated IOP was 22.0 ± 3.1 mmHg on 1.2 ± 0.4 medications, and mean unmedicated IOP was 26.4 ± 2.4 mmHg. Postoperatively, among eyes without later cataract surgery, mean medicated IOP at all visits through 48 months was ≤ 13.7 mmHg (≥ 37% reduction), and annual unmedicated IOP was ≤ 18.4 mmHg (reductions of ≥ 30% vs. preoperative unmedicated IOP and ≥ 16% vs. preoperative medicated IOP). At all postoperative visits among eyes without additional surgery or medication, ≥ 91% of eyes had ≥ 20% IOP reduction on one medication versus preoperative medicated IOP. At month 48, 97 and 98% of eyes achieved IOP ≤ 15 and ≤ 18 mmHg, respectively, on one medication. Six eyes required additional medication, no eyes required additional glaucoma surgery, and safety measurements were favorable throughout follow-up.
Conclusion: IOP control was achieved safely with two trabecular micro-bypass stents, one suprachoroidal stent, and postoperative prostaglandin. This microinvasive, ab interno approach introduces a possible new treatment option for refractory disease.
Trial registration: NCT01456390.
Funding: Glaukos Corporation.
Keywords: Glaucoma; Microinvasive glaucoma surgery (MIGS); Ophthalmology; Prostaglandin; Refractory glaucoma; Suprachoroidal; Trabecular; iStent; iStent Supra.
Figures


Similar articles
-
Five-Year Outcomes Prospective Study of Two First-Generation Trabecular Micro-Bypass Stents (iStent®) in Open-Angle Glaucoma.Curr Eye Res. 2021 Feb;46(2):224-231. doi: 10.1080/02713683.2020.1795881. Epub 2020 Jul 25. Curr Eye Res. 2021. PMID: 32715828 Clinical Trial.
-
Prospective, Randomized, Controlled Pivotal Trial of an Ab Interno Implanted Trabecular Micro-Bypass in Primary Open-Angle Glaucoma and Cataract: Two-Year Results.Ophthalmology. 2019 Jun;126(6):811-821. doi: 10.1016/j.ophtha.2019.03.006. Epub 2019 Mar 14. Ophthalmology. 2019. PMID: 30880108 Clinical Trial.
-
iStent inject trabecular micro-bypass stents with topical prostaglandin as standalone treatment for open-angle glaucoma: 4-year outcomes.Clin Exp Ophthalmol. 2020 Aug;48(6):767-774. doi: 10.1111/ceo.13763. Epub 2020 May 12. Clin Exp Ophthalmol. 2020. PMID: 32311201
-
Ab interno trabecular bypass surgery with Schlemm´s canal microstent (Hydrus) for open angle glaucoma.Cochrane Database Syst Rev. 2020 Mar 9;3(3):CD012740. doi: 10.1002/14651858.CD012740.pub2. Cochrane Database Syst Rev. 2020. PMID: 32147807 Free PMC article.
-
Ab interno trabecular bypass surgery with iStent for open-angle glaucoma.Cochrane Database Syst Rev. 2019 Mar 28;3(3):CD012743. doi: 10.1002/14651858.CD012743.pub2. Cochrane Database Syst Rev. 2019. PMID: 30919929 Free PMC article.
Cited by
-
Microinvasive glaucoma surgery: a review and classification of implant-dependent procedures and techniques.Acta Ophthalmol. 2022 Mar;100(2):e327-e338. doi: 10.1111/aos.14906. Epub 2021 May 14. Acta Ophthalmol. 2022. PMID: 33988310 Free PMC article. Review.
-
Intraocular Pressure Reduction by Femtosecond Laser Created Trabecular Channels in Perfused Human Anterior Segments.Transl Vis Sci Technol. 2021 Aug 2;10(9):22. doi: 10.1167/tvst.10.9.22. Transl Vis Sci Technol. 2021. PMID: 34406341 Free PMC article.
-
Long-term outcome of supraciliary gold micro shunt in refractory glaucoma.Acta Ophthalmol. 2022 May;100(3):e753-e759. doi: 10.1111/aos.14989. Epub 2021 Jul 27. Acta Ophthalmol. 2022. PMID: 34318992 Free PMC article.
-
iStent Trabecular Microbypass Stent Implantation with Phacoemulsification in Patients with Open-Angle Glaucoma: 6-Year Outcomes.Clin Ophthalmol. 2020 Jul 2;14:1859-1866. doi: 10.2147/OPTH.S247910. eCollection 2020. Clin Ophthalmol. 2020. PMID: 32669832 Free PMC article.
-
The effectiveness and safety of one-stage iStent-based micro-invasive glaucoma surgery-A retrospective study.Front Med (Lausanne). 2023 Nov 23;10:1273889. doi: 10.3389/fmed.2023.1273889. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38076245 Free PMC article.
References
-
- American Academy of Ophthalmology. Eye disease statistics, 2016. https://www.aao.org/eye-disease-statistics. Accessed 30 Nov 2016.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

