Random duodenal biopsy to exclude coeliac disease as a cause of anaemia is not cost-efective and should be replaced with universally performed pre-endoscopy serology in patients on a suspected cancer pathway
- PMID: 29476446
- PMCID: PMC5846821
- DOI: 10.1007/s10151-018-1756-7
Random duodenal biopsy to exclude coeliac disease as a cause of anaemia is not cost-efective and should be replaced with universally performed pre-endoscopy serology in patients on a suspected cancer pathway
Abstract
Background: Random duodenal biopsy to exclude coeliac disease during upper gastrointestinal endoscopy for the investigation of iron deficiency anaemia remains a common procedure, but is expensive and time-consuming. Serological investigation for coeliac disease is also recommended, having excellent accuracy with the added benefit of lower cost. This study sought to examine the utility of duodenal biopsy and coeliac serology in the diagnosis of coeliac disease.
Methods: A prospectively maintained database was interrogated to identify all patients having upper gastrointestinal endoscopy for the investigation of anaemia between January 01, 2016, and December 31, 2016.
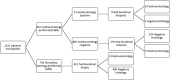
Results: Of the 1131 patients having an endoscopy, coeliac serology was measured in only 412 (36%) and was positive in 9 cases (2%), leading to 6 histological diagnoses of coeliac disease and 3 false positives. Two-hundred and seventy-four patients with negative serology had biopsies taken which were all negative. Only 2/451 (0.4%) patients who had biopsies performed in the absence of a serology test were histologically positive for coeliac disease. The cost per diagnosis of a case of coeliac disease in those with either negative or absent coeliac serology was £18,839 (US$25,244, €21,196).
Conclusions: Random duodenal biopsy is not a cost-effective method of diagnosing coeliac disease and should be replaced with pre-endoscopy coeliac serology.
Keywords: Anaemia; Biopsy; Celiac disease; Colorectal cancer; Endoscopy; Iron deficiency; Serologic tests.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was registered as a service evaluation with the hospital endoscopy department.
Informed consent
This was a retrospective service evaluation, thus consent was not required.
Figures
References
-
- The Association of Coloproctology of Great Britain and Ireland, British Society of Gastroenterology, Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (2013) Guidance on the indications for diagnostic upper GI endoscopy, flexible sigmoidoscopy and colonoscopy. http://www.bsg.org.uk/images/stories/docs/clinical/guidance/indications_.... Accessed 7 Jul 2017
-
- National Institute of Health (2008) Burden of digestive diseases in the United States report. https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/Pages/burd.... Accessed 7 Jul 2017
-
- JAG (2016) Joint advisory group on GI endoscopy 2016 GRS census—analysis of responses NHS acute units in England background. http://www.thejag.org.uk/downloads/National. Policies and reports/JAG GRS census 2016—analysis of service information_v1.zip. Accessed 7 Jul 2017
-
- NICE (2017) Suspected cancer recognition and referral: primary care investigation findings. http://pathways.nice.org.uk/pathways/suspected-cancer-recognition-and-re.... Accessed 19 Oct 2017
-
- Voutilainen ME, Juhola MT. Evaluation of the diagnostic accuracy of gastroscopy to detect gastric tumours: clinicopathological features and prognosis of patients with gastric cancer missed on endoscopy. Eur J Gastroenterol Hepatol. 2005;17:1345–1349. doi: 10.1097/00042737-200512000-00013. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical