Crystal structures of sampatrilat and sampatrilat-Asp in complex with human ACE - a molecular basis for domain selectivity
- PMID: 29476645
- PMCID: PMC5947662
- DOI: 10.1111/febs.14421
Crystal structures of sampatrilat and sampatrilat-Asp in complex with human ACE - a molecular basis for domain selectivity
Abstract
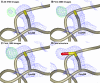
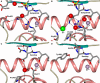
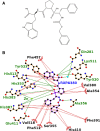
Angiotensin-1-converting enzyme (ACE) is a zinc metallopeptidase that consists of two homologous catalytic domains (known as nACE and cACE) with different substrate specificities. Based on kinetic studies it was previously reported that sampatrilat, a tight-binding inhibitor of ACE, Ki = 13.8 nm and 171.9 nm for cACE and nACE respectively [Sharma et al., Journal of Chemical Information and Modeling (2016), 56, 2486-2494], was 12.4-fold more selective for cACE. In addition, samAsp, in which an aspartate group replaces the sampatrilat lysine, was found to be a nonspecific and lower micromolar affinity inhibitor. Here, we report a detailed three-dimensional structural analysis of sampatrilat and samAsp binding to ACE using high-resolution crystal structures elucidated by X-ray crystallography, which provides a molecular basis for differences in inhibitor affinity and selectivity for nACE and cACE. The structures show that the specificity of sampatrilat can be explained by increased hydrophobic interactions and a H-bond from Glu403 of cACE with the lysine side chain of sampatrilat that are not observed in nACE. In addition, the structures clearly show a significantly greater number of hydrophilic and hydrophobic interactions with sampatrilat compared to samAsp in both cACE and nACE consistent with the difference in affinities. Our findings provide new experimental insights into ligand binding at the active site pockets that are important for the design of highly specific domain selective inhibitors of ACE.
Database: The atomic coordinates and structure factors for N- and C-domains of ACE bound to sampatrilat and sampatrilat-Asp complexes (6F9V, 6F9R, 6F9T and 6F9U respectively) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
Keywords: angiotensin-1-converting enzyme; crystallography; domain specificity; enzyme kinetics; enzyme structure; metalloprotease; sampatrilat.
© 2018 The Authors. The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures



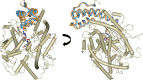

References
-
- Yang HY, Erdos EG & Levin Y (1970) A dipeptidyl carboxypeptidase that converts angiotensin I and inactivates bradykinin. Biochim Biophys Acta 214, 374–376. - PubMed
-
- Araujo MC, Melo RL, Cesari MH, Juliano MA, Juliano L & Carmona AK (2000) Peptidase specificity characterization of C‐ and N‐terminal catalytic sites of angiotensin I‐converting enzyme. Biochemistry 39, 8519–8525. - PubMed
-
- Rousseau A, Michaud A, Chauvet MT, Lenfant M & Corvol P (1995) The hemoregulatory peptide N‐acetyl‐Ser‐Asp‐Lys‐Pro is a natural and specific substrate of the N‐terminal active site of human angiotensin‐converting enzyme. J Biol Chem 270, 3656–3661. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

