A Conserved Distal Lung Regenerative Pathway in Acute Lung Injury
- PMID: 29476724
- PMCID: PMC5906746
- DOI: 10.1016/j.ajpath.2018.01.021
A Conserved Distal Lung Regenerative Pathway in Acute Lung Injury
Abstract
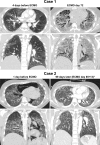
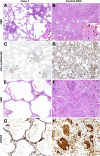
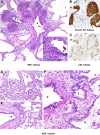
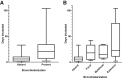
Improved tools have led to a burgeoning understanding of lung regeneration in mice, but it is not yet known how these insights may be relevant to acute lung injury in humans. We report in detail two cases of fulminant idiopathic acute lung injury requiring extracorporeal membrane oxygenation in previously healthy young adults with acute respiratory distress syndrome, one of whom required lung transplantation. Biopsy specimens showed diffuse alveolar injury with a striking paucity of alveolar epithelial regeneration, rare hyaline membranes, and diffuse contiguous airspace lining by macrophages. This novel constellation was termed diffuse alveolar injury with delayed epithelization. In addition, mirroring data from murine models of lung injury/regeneration, peribronchiolar basaloid pods (previously described as squamous metaplasia) and ciliated bronchiolarization were identified in these patients and in 39% of 57 historical cases with diffuse alveolar damage. These findings demonstrate a common and clinically relevant human disease correlate for murine models of severe acute lung injury. Evidence suggests that peribronchiolar basaloid pods and bronchiolarization are related spatially and temporally and likely represent overlapping sequential stages of the response to severe distal airway injury.
Copyright © 2018 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Cardinal-Fernandez P., Bajwa E.K., Dominguez-Calvo A., Menendez J.M., Papazian L., Thompson B.T. The presence of diffuse alveolar damage on open lung biopsy is associated with mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Chest. 2016;149:1155–1164. - PubMed
-
- Papazian L., Doddoli C., Chetaille B., Gernez Y., Thirion X., Roch A., Donati Y., Bonnety M., Zandotti C., Thomas P. A contributive result of open-lung biopsy improves survival in acute respiratory distress syndrome patients. Crit Care Med. 2007;35:755–762. - PubMed
-
- Patel S.R., Karmpaliotis D., Ayas N.T., Mark E.J., Wain J., Thompson B.T., Malhotra A. The role of open-lung biopsy in ARDS. Chest. 2004;125:197–202. - PubMed
-
- Thompson B.T., Guerin C., Esteban A. Should ARDS be renamed diffuse alveolar damage? Intensive Care Med. 2016;42:653–655. - PubMed
-
- Katzenstein A.-L.A. Katzenstein and Askin's surgical pathology of non-neoplastic lung disease. In: Katzenstein A.-L.A., Askin F.B., Livolsi V.A., editors. Major Problems in Pathology. ed 4. Saunders/Elsevier; Philadelphia: 2006.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

