Enhancing endogenous capacity to repair a stroke-damaged brain: An evolving field for stroke research
- PMID: 29476785
- PMCID: PMC6075953
- DOI: 10.1016/j.pneurobio.2018.01.004
Enhancing endogenous capacity to repair a stroke-damaged brain: An evolving field for stroke research
Abstract
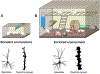
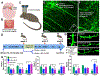
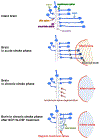
Stroke represents a severe medical condition that causes stroke survivors to suffer from long-term and even lifelong disability. Over the past several decades, a vast majority of stroke research targets neuroprotection in the acute phase, while little work has been done to enhance stroke recovery at the later stage. Through reviewing current understanding of brain plasticity, stroke pathology, and emerging preclinical and clinical restorative approaches, this review aims to provide new insights to advance the research field for stroke recovery. Lifelong brain plasticity offers the long-lasting possibility to repair a stroke-damaged brain. Stroke impairs the structural and functional integrity of entire brain networks; the restorative approaches containing multi-components have great potential to maximize stroke recovery by rebuilding and normalizing the stroke-disrupted entire brain networks and brain functioning. The restorative window for stroke recovery is much longer than previously thought. The optimal time for brain repair appears to be at later stage of stroke rather than the earlier stage. It is expected that these new insights will advance our understanding of stroke recovery and assist in developing the next generation of restorative approaches for enhancing brain repair after stroke.
Keywords: Brain plasticity; Brain repair; Restorative timing; Stroke recovery.
Published by Elsevier Ltd.
Figures

References
-
- Allen NJ, Barres BA, 2005. Signaling between glia and neurons: focus on synaptic plasticity. Curr. Opin. Neurobiol 15, 542–548. - PubMed
-
- Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, Carvalho RF, Carvalho AP, Duarte CB, 2005. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ 12, 1329–1343. - PubMed
-
- Altman J, Das GD, 1964. Autoradiographic examination of the effects of enriched environment on the rate of glial multiplication in the adult rat brain. Nature 204, 1161–1163. - PubMed
-
- Altschuler EL, Wisdom SB, Stone L, Foster C, Galasko D, Llewellyn DME, Ramachandran VS, 1999. Rehabilitation of hemiparesis after stroke with a mirror. Lancet 353, 2035–2036. - PubMed
-
- Andersen KK, Olsen TS, Dehlendorff C, Kammersgaard LP, 2009. Hemorrhagic and ischemic strokes compared: stroke severity mortality, and risk factors. Stroke 40, 2068–2072. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

