Assessing complex movement behaviors in rodent models of neurological disorders
- PMID: 29476821
- PMCID: PMC6103896
- DOI: 10.1016/j.nlm.2018.02.025
Assessing complex movement behaviors in rodent models of neurological disorders
Abstract
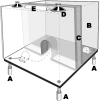
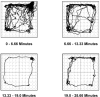
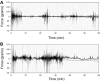
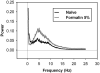
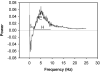
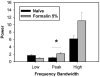
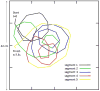
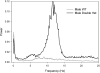
Behavioral phenotyping is a crucial step in validating animal models of human disease. Most traditional behavioral analyses rely on investigator observation of animal subjects, which can be confounded by inter-observer variability, scoring consistency, and the ability to observe extremely rapid, small, or repetitive movements. Force-Plate Actimeter (FPA)-based assessments can quantify locomotor activity and detailed motor activity with an incredibly rich data stream that can reveal details of movement unobservable by the naked eye. This report describes four specific examples of FPA analysis of behavior that have been useful in specific rat or mouse models of human neurological disease, which show how FPA analysis can be used to capture and quantify specific features of the complex behavioral phenotypes of these animal models. The first example quantifies nociceptive behavior of the rat following injection of formalin into the footpad as a common model of persistent inflammatory pain. The second uses actimetry to quantify intense, rapid circling behaviors in a transgenic mouse that overexpresses human laminin α5, a basement membrane protein. The third example assesses place preference behaviors in a rat model of migraine headache modeling phonophobia and photophobia. In the fourth example, FPA analysis revealed a unique movement signature emerged with age in a digenic mutant mouse model of Tourette Syndrome. Taken together, these approaches demonstrate the power and usefulness of the FPA in the examination and quantification of minute details of motor behaviors, greatly expanding the scope and detail of behavioral phenotyping of preclinical models of human disease.
Keywords: Force-plate Actimeter; Laminin; Locomotion; Migraine; Open field; Tremor.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
No competing financial interests exist for any of the authors of this manuscript.
Figures




Similar articles
-
Behavioral and cognitive animal models in headache research.J Headache Pain. 2019 Jan 31;20(1):11. doi: 10.1186/s10194-019-0963-6. J Headache Pain. 2019. PMID: 30704400 Free PMC article. Review.
-
A force-plate actometer for quantitating rodent behaviors: illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor.J Neurosci Methods. 2001 May 30;107(1-2):107-24. doi: 10.1016/s0165-0270(01)00359-4. J Neurosci Methods. 2001. PMID: 11389948
-
Use of a force-sensing automated open field apparatus in a longitudinal study of multiple behavioral deficits in CAG140 Huntington's disease model mice.Behav Brain Res. 2015 Nov 1;294:7-16. doi: 10.1016/j.bbr.2015.07.036. Epub 2015 Jul 22. Behav Brain Res. 2015. PMID: 26210937 Free PMC article.
-
A novel black-hooded mutant rat (ci3) with spontaneous circling behavior but normal auditory and vestibular functions.Neuroscience. 2001;107(4):615-28. doi: 10.1016/s0306-4522(01)00390-6. Neuroscience. 2001. PMID: 11720785
-
Movement Quantification in Neurological Diseases: Methods and Applications.IEEE Rev Biomed Eng. 2016;9:15-31. doi: 10.1109/RBME.2016.2543683. Epub 2016 Mar 17. IEEE Rev Biomed Eng. 2016. PMID: 27008673 Review.
Cited by
-
Altered synaptic glutamate homeostasis contributes to cognitive decline in young APP/PSEN1 mice.Neurobiol Dis. 2021 Oct;158:105486. doi: 10.1016/j.nbd.2021.105486. Epub 2021 Aug 24. Neurobiol Dis. 2021. PMID: 34450329 Free PMC article.
-
Behavioral and cognitive animal models in headache research.J Headache Pain. 2019 Jan 31;20(1):11. doi: 10.1186/s10194-019-0963-6. J Headache Pain. 2019. PMID: 30704400 Free PMC article. Review.
References
-
- The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;(24 Suppl 1):9–160. - PubMed
-
- Abbott FV, Ocvirk R, Najafee R, Franklin KB. Improving the efficiency of the formalin test. Pain. 1999;83(3):561–569. - PubMed
-
- Allen AL, McCarson KE. Estrogen increases nociception-evoked brain-derived neurotrophic factor gene expression in the female rat. Neuroendocrinology. 2005;81(3):193–199. - PubMed
-
- Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004;55(1):27–36. - PubMed
-
- Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79(2):964–982. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

