Strategies to improve the regulatory assessment of developmental neurotoxicity (DNT) using in vitro methods
- PMID: 29476865
- PMCID: PMC6095942
- DOI: 10.1016/j.taap.2018.02.008
Strategies to improve the regulatory assessment of developmental neurotoxicity (DNT) using in vitro methods
Abstract
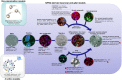
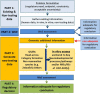
Currently, the identification of chemicals that have the potential to induce developmental neurotoxicity (DNT) is based on animal testing. Since at the regulatory level, systematic testing of DNT is not a standard requirement within the EU or USA chemical legislation safety assessment, DNT testing is only performed in higher tiered testing triggered based on chemical structure activity relationships or evidence of neurotoxicity in systemic acute or repeated dose toxicity studies. However, these triggers are rarely used and, in addition, do not always serve as reliable indicators of DNT, as they are generally based on observations in adult rodents. Therefore, there is a pressing need for developing alternative methodologies that can reliably support identification of DNT triggers, and more rapidly and cost-effectively support the identification and characterization of chemicals with DNT potential. We propose to incorporate mechanistic knowledge and data derived from in vitro studies to support various regulatory applications including: (a) the identification of potential DNT triggers, (b) initial chemical screening and prioritization, (c) hazard identification and characterization, (d) chemical biological grouping, and (e) assessment of exposure to chemical mixtures. Ideally, currently available cellular neuronal/glial models derived from human induced pluripotent stem cells (hiPSCs) should be used as they allow evaluation of chemical impacts on key neurodevelopmental processes, by reproducing different windows of exposure during human brain development. A battery of DNT in vitro test methods derived from hiPSCs could generate valuable mechanistic data, speeding up the evaluation of thousands of compounds present in industrial, agricultural and consumer products that lack safety data on DNT potential.
Keywords: Adverse outcome pathways; Developmental neurotoxicity; Human in vitro test systems; Integrated Approaches to Testing and Assessment; Regulatory purposes.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- (NRC), N.R.C . Committee on Pesticides in the Diets of Infants and Children. National Academy Press; Washington, DC: 1993. Pesticides in the diets of infants and children.
-
- (NRC), N.R.C . National Academy Press; Washington, DC: 2007. Toxicity Testing in the 21st Century: A Vision and a Strategy.
-
- Adinolfi M. The development of the human blood-CSF-brain barrier. Dev. Med. Child Neurol. 1985;27:532–537. - PubMed
-
- Ankley G.T., Bennett R.S., Erickson R.J., Hoff D.J., Hornung M.W., Johnson R.D., Mount D.R., Nichols J.W., Russom C.L., Schmieder P.K., Serrrano J.A., Tietge J.E., Villeneuve D.L. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010;29:730–741. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

