In vivo maternal and in vitro BPA exposure effects on hypothalamic neurogenesis and appetite regulators
- PMID: 29476947
- PMCID: PMC8085909
- DOI: 10.1016/j.envres.2018.02.011
In vivo maternal and in vitro BPA exposure effects on hypothalamic neurogenesis and appetite regulators
Abstract
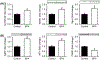
In utero exposure to the ubiquitous plasticizer, bisphenol A (BPA) is associated with offspring obesity. As food intake/appetite is one of the critical elements contributing to obesity, we determined the effects of in vivo maternal BPA and in vitro BPA exposure on newborn hypothalamic stem cells which form the arcuate nucleus appetite center. For in vivo studies, female rats received BPA prior to and during pregnancy via drinking water, and newborn offspring primary hypothalamic neuroprogenitor (NPCs) were obtained and cultured. For in vitro BPA exposure, primary hypothalamic NPCs from healthy newborns were utilized. In both cases, we studied the effects of BPA on NPC proliferation and differentiation, including putative signal and appetite factors. Maternal BPA increased hypothalamic NPC proliferation and differentiation in newborns, in conjunction with increased neuroproliferative (Hes1) and proneurogenic (Ngn3) protein expression. With NPC differentiation, BPA exposure increased appetite peptide and reduced satiety peptide expression. In vitro BPA-treated control NPCs showed results that were consistent with in vivo data (increase appetite vs satiety peptide expression) and further showed a shift towards neuronal versus glial fate as well as an increase in the epigenetic regulator lysine-specific histone demethylase1 (LSD1). These findings emphasize the vulnerability of stem-cell populations that are involved in life-long regulation of metabolic homeostasis to epigenetically-mediated endocrine disruption by BPA during early life.
Keywords: Epigenetic; Neuroprogenitor cells; Obesity; Perinatal exposures; Proliferation, differentiation.
Copyright © 2018. Published by Elsevier Inc.
Figures



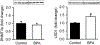
References
-
- Adamo A, et al. , 2011. LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nat.Cell Biol 13, 652–659. - PubMed
-
- Barker JM, Galea LA, 2008. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience. 152, 888–902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

