Evaluation of the neonatal streptozotocin model of diabetes in rats: Evidence for a model of neuropathic pain
- PMID: 29477037
- PMCID: PMC5851873
- DOI: 10.1016/j.pharep.2017.09.002
Evaluation of the neonatal streptozotocin model of diabetes in rats: Evidence for a model of neuropathic pain
Abstract
Background: The purpose of this study was to evaluate the participation of satellite glial cells (SGC), microglia and astrocytes in a model of streptozotocin-induced diabetes initiated in neonatal rats (nSTZ) and to determine the pharmacological profile for pain relief.
Methods: nSTZ was used to induce experimental diabetes. Von Frey filaments were used to assess tactile allodynia. Drugs were given by systemic administration. Western blotting and immunohistochemistry were used to determine protein expression and cellular localization.
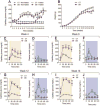
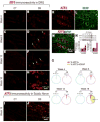
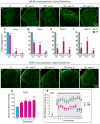
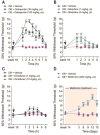
Results: nSTZ produced mild hyperglycemia, weight loss, glucose intolerance, and reduction of nerve conduction velocity of C fibers. Moreover, nSTZ enhanced activating transcription factor 3 (ATF3) immunoreactivity in dorsal root ganglia (DRG) and sciatic nerve of adult rats. ATF3 was found in SGC (GFAP+ cells) surrounding DRG at week 16. Late changes in ATF3 immunoreactivity in DRG correlated with up-regulation of ATF3 and GFAP protein expression. nSTZ increased GFAP and OX-42 immunoreactivity and percentage of hypertrophied and ameboid microglia in the spinal dorsal horn. These changes correlated with the presence of mechanical hypersensitivity (tactile allodynia). Administration of gabapentin (30-100mg/kg, po) and metformin (200mg/kg/day, po for 2 weeks) alleviated tactile allodynia, whereas morphine (1-3mg/kg, ip) had a modest effect.
Conclusions: Results suggest that nSTZ leads to activation of SGC, microglia and astrocytes in DRG and spinal cord. Pharmacological profile in the nSTZ model resembles diabetic neuropathic pain in humans. Our findings support the conclusion that the nSTZ rat model has utility for the study of a long-lasting diabetic neuropathic pain.
Keywords: Astrocytes; Diabetes; Microglia; Satellite glial cells; nSTZ model.
Copyright © 2017 Institute of Pharmacology, Polish Academy of Sciences. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
None.
Figures

Comment in
-
Neuropathic pain models and outcome measures: a dual translational challenge.Ann Transl Med. 2018 Nov;6(Suppl 1):S42. doi: 10.21037/atm.2018.09.58. Ann Transl Med. 2018. PMID: 30613617 Free PMC article. No abstract available.
Similar articles
-
Gabapentin reverses microglial activation in the spinal cord of streptozotocin-induced diabetic rats.Eur J Pain. 2009 Sep;13(8):807-11. doi: 10.1016/j.ejpain.2008.09.010. Epub 2008 Oct 31. Eur J Pain. 2009. PMID: 18977160
-
Diabetes-induced Neuropathic Mechanical Hyperalgesia Depends on P2X4 Receptor Activation in Dorsal Root Ganglia.Neuroscience. 2019 Feb 1;398:158-170. doi: 10.1016/j.neuroscience.2018.12.003. Epub 2018 Dec 8. Neuroscience. 2019. PMID: 30537520
-
Effect of levetiracetam versus gabapentin on peripheral neuropathy and sciatic degeneration in streptozotocin-diabetic mice: Influence on spinal microglia and astrocytes.Eur J Pharmacol. 2016 Jan 15;771:162-72. doi: 10.1016/j.ejphar.2015.12.035. Epub 2015 Dec 19. Eur J Pharmacol. 2016. PMID: 26712375
-
Novel Nanotechnological Approaches for Targeting Dorsal Root Ganglion (DRG) in Mitigating Diabetic Neuropathic Pain (DNP).Front Endocrinol (Lausanne). 2022 Feb 8;12:790747. doi: 10.3389/fendo.2021.790747. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35211091 Free PMC article. Review.
-
Spinal neuron-glial crosstalk and ion channel dysregulation in diabetic neuropathic pain.Front Immunol. 2025 Apr 8;16:1480534. doi: 10.3389/fimmu.2025.1480534. eCollection 2025. Front Immunol. 2025. PMID: 40264787 Free PMC article. Review.
Cited by
-
Cilostazol Ameliorates Peripheral Neuropathic Pain in Streptozotocin-Induced Type I Diabetic Rats.Front Pharmacol. 2022 Jan 18;12:771271. doi: 10.3389/fphar.2021.771271. eCollection 2021. Front Pharmacol. 2022. PMID: 35115925 Free PMC article.
-
Neuropathic pain models and outcome measures: a dual translational challenge.Ann Transl Med. 2018 Nov;6(Suppl 1):S42. doi: 10.21037/atm.2018.09.58. Ann Transl Med. 2018. PMID: 30613617 Free PMC article. No abstract available.
-
Metformin: A Prospective Alternative for the Treatment of Chronic Pain.Front Pharmacol. 2020 Sep 23;11:558474. doi: 10.3389/fphar.2020.558474. eCollection 2020. Front Pharmacol. 2020. PMID: 33178015 Free PMC article. Review.
-
Minocycline reduces proinflammatory and oxidative stress markers in the spinal cord and morphology changes in sciatic nerve of Type 2 diabetic neuropathy rat model.Diabetol Int. 2025 Mar 19;16(3):483-492. doi: 10.1007/s13340-025-00811-3. eCollection 2025 Jul. Diabetol Int. 2025. PMID: 40607152
-
Syringic Acid Ameliorates Cardiac, Hepatic, Renal and Neuronal Damage Induced by Chronic Hyperglycaemia in Wistar Rats: A Behavioural, Biochemical and Histological Analysis.Molecules. 2022 Oct 9;27(19):6722. doi: 10.3390/molecules27196722. Molecules. 2022. PMID: 36235257 Free PMC article.
References
-
- Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9:36–45. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
