Japanese multicenter database of healthy controls for [123I]FP-CIT SPECT
- PMID: 29478082
- PMCID: PMC5993845
- DOI: 10.1007/s00259-018-3976-5
Japanese multicenter database of healthy controls for [123I]FP-CIT SPECT
Abstract
Purpose: The aim of this multicenter trial was to generate a [123I]FP-CIT SPECT database of healthy controls from the common SPECT systems available in Japan.
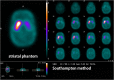
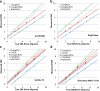
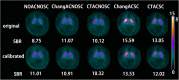
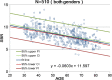
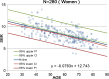
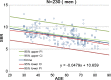
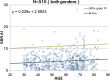
Methods: This study included 510 sets of SPECT data from 256 healthy controls (116 men and 140 women; age range, 30-83 years) acquired from eight different centers. Images were reconstructed without attenuation or scatter correction (NOACNOSC), with only attenuation correction using the Chang method (ChangACNOSC) or X-ray CT (CTACNOSC), and with both scatter and attenuation correction using the Chang method (ChangACSC) or X-ray CT (CTACSC). These SPECT images were analyzed using the Southampton method. The outcome measure was the specific binding ratio (SBR) in the striatum. These striatal SBRs were calibrated from prior experiments using a striatal phantom.
Results: The original SBRs gradually decreased in the order of ChangACSC, CTACSC, ChangACNOSC, CTACNOSC, and NOACNOSC. The SBRs for NOACNOSC were 46% lower than those for ChangACSC. In contrast, the calibrated SBRs were almost equal under no scatter correction (NOSC) conditions. A significant effect of age was found, with an SBR decline rate of 6.3% per decade. In the 30-39 age group, SBRs were 12.2% higher in women than in men, but this increase declined with age and was absent in the 70-79 age group.
Conclusions: This study provided a large-scale quantitative database of [123I]FP-CIT SPECT scans from different scanners in healthy controls across a wide age range and with balanced sex representation. The phantom calibration effectively harmonizes SPECT data from different SPECT systems under NOSC conditions. The data collected in this study may serve as a reference database.
Keywords: Dopamine transporter; Multicenter trial; Normal database; SPECT; [123I]FP-CIT.
Conflict of interest statement
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Conflict of interest
H. Matsuda has received a research grant and speaker honorarium from Nihon Medi-Physics Co., Ltd.. M. Murata, K. Sako, H. Toyama, Y. Taki, H. Nagayama, K. Ono, A. Kono, S. Hirano, N. Sato, H. Takano and J. Hatazawa have received research grants from Nihon Medi-Physics Co., Ltd.. Y. Mukai, H. Ono, Y. Inui, H, Shimomura, A. Tateno, H. Murakami, S. Kuwabara, N. Maikusa, M. Ogawa, E. Imabayashi, and R. Takahashi have no conflicts of interest to declare.
Figures
References
-
- Booij J, Tissingh G, Boer GJ, Speelman JD, Stoof JC, Janssen AG, et al. [123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson's disease. J Neurol Neurosurg Psychiatry. 1997;62(2):133–140. doi: 10.1136/jnnp.62.2.133. - DOI - PMC - PubMed
-
- Albert NL, Unterrainer M, Diemling M, Xiong G, Bartenstein P, Koch W, et al. Implementation of the European multicentre database of healthy controls for [(123)I]FP-CIT SPECT increases diagnostic accuracy in patients with clinically uncertain parkinsonian syndromes. Eur J Nucl Med Mol Imaging. 2016;43(7):1315–1322. doi: 10.1007/s00259-015-3304-2. - DOI - PubMed
-
- Meyer PT, Sattler B, Lincke T, Seese A, Sabri O. Investigating dopaminergic neurotransmission with 123I-FP-CIT SPECT: comparability of modern SPECT systems. J Nucl Med. 2003;44(5):839–845. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

