PD-L1 inhibition with avelumab for metastatic Merkel cell carcinoma
- PMID: 29478343
- PMCID: PMC6360093
- DOI: 10.1080/17512433.2018.1445966
PD-L1 inhibition with avelumab for metastatic Merkel cell carcinoma
Abstract
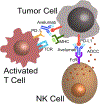
Merkel cell carcinoma (MCC) is a rare and aggressive neuroendocrine skin cancer that lacks durable responses to traditional chemotherapy. Areas covered: After MCC was shown to be an immunogenic tumor, small trials revealed high objective response rates to PD-1/PD-L1 checkpoint inhibitors. The JAVELIN Merkel 200 (NCT02155647) trial tested the use of avelumab, a human IgG1 monoclonal antibody against PD-L1, in metastatic MCC. Avelumab recently became the first approved drug for metastatic MCC. Expert commentary: By conducting broad phase I studies assessing the safety of avelumab and a small phase II study demonstrating efficacy in this rare orphan tumor type, avelumab gained accelerated approval for the treatment of metastatic MCC. Additional studies are needed to determine how the antibody-dependent cellular cytotoxicity (ADCC) competent Fc region of avelumab contributes to disease control. Remaining questions: Longer follow-up will determine the durability of checkpoint blockade in controlling metastatic MCC. Additional studies will assess the utility and safety of adjuvant checkpoint blockade in patients with excised MCC. How to increase response rates by combining PD-1/PD-L1 blockade with other treatment approaches needs to be explored. In addition, treatment options for MCC patients who fail or do not respond to avelumab need to be identified.
Keywords: Anti-PD-1; Bavencio; Merkel cell carcinoma; anti-PD-L1; avelumab; checkpoint inhibitor; immunotherapy; pembrolizumab.
Conflict of interest statement
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Figures
References
-
- Schrama D, Becker JC. Merkel cell carcinoma--pathogenesis, clinical aspects and treatment. J Eur Acad Dermatol Venereol. 2011. October;25(10):1121–9. - PubMed
-
- Nicolaidou E, Mikrova A, Antoniou C, et al. Advances in Merkel cell carcinoma pathogenesis and management: a recently discovered virus, a new international consensus staging system and new diagnostic codes. Br J Dermatol. 2012. January;166(1):16–21. - PubMed
-
- Lebbe C, Becker JC, Grob JJ, et al. Diagnosis and treatment of Merkel Cell Carcinoma. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015. November;51(16):2396–403. - PubMed
-
- Fitzgerald TL, Dennis S, Kachare SD, et al. Dramatic Increase in the Incidence and Mortality from Merkel Cell Carcinoma in the United States. Am Surg. 2015. August;81(8):802–6. - PubMed
-
- Youlden DR, Soyer HP, Youl PH, et al. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993-2010. JAMA Dermatol. 2014. August;150(8):864–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials