Pre-existing Immunity to Oncolytic Virus Potentiates Its Immunotherapeutic Efficacy
- PMID: 29478729
- PMCID: PMC6079372
- DOI: 10.1016/j.ymthe.2018.01.019
Pre-existing Immunity to Oncolytic Virus Potentiates Its Immunotherapeutic Efficacy
Abstract
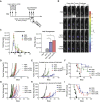
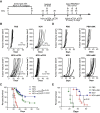
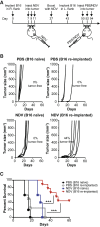
Anti-viral immunity presents a major hurdle for systemically administered oncolytic viruses (OV). Intratumoral OV therapy has a potential to overcome this problem through activation of anti-tumor immune response, with local and abscopal effects. However, the effects of anti-viral immunity in such a setting are still not well defined. Using Newcastle Disease Virus (NDV) as a model, we explore the effects of pre-existing anti-viral immunity on therapeutic efficacy in syngeneic mouse tumor models. Unexpectedly, we find that while pre-existing immunity to NDV limits its replication in tumors, tumor clearance, abscopal anti-tumor immune effects, and survival are not compromised and, on the contrary, are superior in NDV-immunized mice. These findings demonstrate that pre-existing immunity to NDV may increase its therapeutic efficacy through potentiation of systemic anti-tumor immunity, which provides clinical rationale for repeated therapeutic dosing and prompts investigation of such effects with other OVs.
Keywords: NDV; Newcastle Disease Virus; cancer immunotherapy; immuno-oncology; oncolytic virus; pre-existing immunity; tumor immunology.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



Comment in
-
The Immune System in Oncolytic Immunovirotherapy: Gospel, Schism and Heresy.Mol Ther. 2018 Apr 4;26(4):942-946. doi: 10.1016/j.ymthe.2018.03.007. Epub 2018 Mar 21. Mol Ther. 2018. PMID: 29573976 Free PMC article. No abstract available.
References
-
- Chen Y., Yu D.C., Charlton D., Henderson D.R. Pre-existent adenovirus antibody inhibits systemic toxicity and antitumor activity of CN706 in the nude mouse LNCaP xenograft model: implications and proposals for human therapy. Hum. Gene Ther. 2000;11:1553–1567. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

