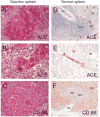
ACE phenotyping in Gaucher disease
- PMID: 29478818
- PMCID: PMC5891352
- DOI: 10.1016/j.ymgme.2018.02.007
ACE phenotyping in Gaucher disease
Abstract
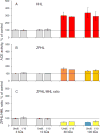
Background: Gaucher disease is characterized by the activation of splenic and hepatic macrophages, accompanied by dramatically increased levels of angiotensin-converting enzyme (ACE). To evaluate the source of the elevated blood ACE, we performed complete ACE phenotyping using blood, spleen and liver samples from patients with Gaucher disease and controls.
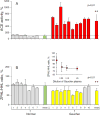
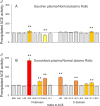
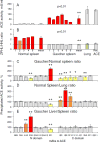
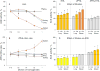
Methods: ACE phenotyping included 1) immunohistochemical staining for ACE; 2) measuring ACE activity with two substrates (HHL and ZPHL); 3) calculating the ratio of the rates of substrate hydrolysis (ZPHL/HHL ratio); 4) assessing the conformational fingerprint of ACE by evaluating the pattern of binding of monoclonal antibodies to 16 different ACE epitopes.
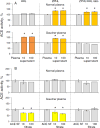
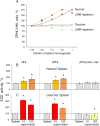
Results: We show that in patients with Gaucher disease, the dramatically increased levels of ACE originate from activated splenic and/or hepatic macrophages (Gaucher cells), and that both its conformational fingerprint and kinetic characteristics (ZPHL/HHL ratio) differ from controls and from patients with sarcoid granulomas. Furthermore, normal spleen was found to produce high levels of endogenous ACE inhibitors and a novel, tightly-bound 10-30 kDa ACE effector which is deficient in Gaucher spleen.
Conclusions: The conformation of ACE is tissue-specific. In Gaucher disease, ACE produced by activated splenic macrophages differs from that in hepatic macrophages, as well as from macrophages and dendritic cells in sarcoid granulomas. The observed differences are likely due to altered ACE glycosylation or sialylation in these diseased organs. The conformational differences in ACE may serve as a specific biomarker for Gaucher disease.
Keywords: ACE inhibitors; Angiotensin I-converting enzyme; CD143; Conformation; Gaucher disease; Monoclonal antibodies; Tissue specificity.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Tissue ACE phenotyping in lung cancer.PLoS One. 2019 Dec 26;14(12):e0226553. doi: 10.1371/journal.pone.0226553. eCollection 2019. PLoS One. 2019. PMID: 31877149 Free PMC article.
-
[Phenotyping of angiotensin-converting enzyme in the prostate in patients with prostate cancer and benign prostatic hyperplasia].Urologiia. 2020 Nov;(5):20-27. Urologiia. 2020. PMID: 33185341 Russian.
-
Immunofluorescent detection of angiotensin-converting enzyme (ACE) in Gaucher cells.Am J Med. 1980 Sep;69(3):408-10. doi: 10.1016/0002-9343(80)90012-1. Am J Med. 1980. PMID: 6251722
-
The significance of angiotensin I converting enzyme in granulomatous inflammation. Functions of ACE in granulomas.Sarcoidosis. 1986 Mar;3(1):19-26. Sarcoidosis. 1986. PMID: 3033785 Review.
-
Angiotensin-converting enzyme activity in sarcoidosis and other disorders.Sarcoidosis. 1985 Mar;2(1):25-34. Sarcoidosis. 1985. PMID: 3031789 Review.
Cited by
-
Macrophage angiotensin-converting enzyme reduces atherosclerosis by increasing peroxisome proliferator-activated receptor α and fundamentally changing lipid metabolism.Cardiovasc Res. 2023 Aug 7;119(9):1825-1841. doi: 10.1093/cvr/cvad082. Cardiovasc Res. 2023. PMID: 37225143 Free PMC article.
-
An observational study to investigate the relationship between plasma glucosylsphingosine (lyso-Gb1) concentration and treatment outcomes of patients with Gaucher disease in Japan.Orphanet J Rare Dis. 2022 Nov 3;17(1):401. doi: 10.1186/s13023-022-02549-6. Orphanet J Rare Dis. 2022. PMID: 36329499 Free PMC article.
-
Overexpression of ACE in Myeloid Cells Increases Immune Effectiveness and Leads to a New Way of Considering Inflammation in Acute and Chronic Diseases.Curr Hypertens Rep. 2020 Jan 8;22(1):4. doi: 10.1007/s11906-019-1008-x. Curr Hypertens Rep. 2020. PMID: 31916032 Free PMC article. Review.
-
Urinary ACE Phenotyping as a Research and Diagnostic Tool: Identification of Sex-Dependent ACE Immunoreactivity.Biomedicines. 2023 Mar 20;11(3):953. doi: 10.3390/biomedicines11030953. Biomedicines. 2023. PMID: 36979933 Free PMC article.
-
Effects of Angiotensin-I-Converting Enzyme (ACE) Mutations Associated with Alzheimer's Disease on Blood ACE Phenotype.Biomedicines. 2024 Oct 21;12(10):2410. doi: 10.3390/biomedicines12102410. Biomedicines. 2024. PMID: 39457722 Free PMC article.
References
-
- Beutler E, Grabowski GA. The Metabolic and Molecular Bases of Inherited Disease III. McGraw-Hill; New York: 2001. Gaucher Diseases; pp. 3635–3668.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

