Remodeling the Specificity of an Endosomal CORVET Tether Underlies Formation of Regulated Secretory Vesicles in the Ciliate Tetrahymena thermophila
- PMID: 29478853
- PMCID: PMC5840023
- DOI: 10.1016/j.cub.2018.01.047
Remodeling the Specificity of an Endosomal CORVET Tether Underlies Formation of Regulated Secretory Vesicles in the Ciliate Tetrahymena thermophila
Abstract
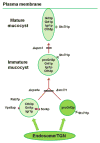
In the endocytic pathway of animals, two related complexes, called CORVET (class C core vacuole/endosome transport) and HOPS (homotypic fusion and protein sorting), act as both tethers and fusion factors for early and late endosomes, respectively. Mutations in CORVET or HOPS lead to trafficking defects and contribute to human disease, including immune dysfunction. HOPS and CORVET are conserved throughout eukaryotes, but remarkably, in the ciliate Tetrahymena thermophila, the HOPS-specific subunits are absent, while CORVET-specific subunits have proliferated. VPS8 (vacuolar protein sorting), a CORVET subunit, expanded to 6 paralogs in Tetrahymena. This expansion correlated with loss of HOPS within a ciliate subgroup, including the Oligohymenophorea, which contains Tetrahymena. As uncovered via forward genetics, a single VPS8 paralog in Tetrahymena (VPS8A) is required to synthesize prominent secretory granules called mucocysts. More specifically, Δvps8a cells fail to deliver a subset of cargo proteins to developing mucocysts, instead accumulating that cargo in vesicles also bearing the mucocyst-sorting receptor Sor4p. Surprisingly, although this transport step relies on CORVET, it does not appear to involve early endosomes. Instead, Vps8a associates with the late endosomal/lysosomal marker Rab7, indicating that target specificity switching occurred in CORVET subunits during the evolution of ciliates. Mucocysts belong to a markedly diverse and understudied class of protist secretory organelles called extrusomes. Our results underscore that biogenesis of mucocysts depends on endolysosomal trafficking, revealing parallels with invasive organelles in apicomplexan parasites and suggesting that a wide array of secretory adaptations in protists, like in animals, depend on mechanisms related to lysosome biogenesis.
Keywords: alveolata; endosomal tether; evolution; extrusome; gene loss; lysosome-related organelle; membrane trafficking; protist.
Crown Copyright © 2018. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
An endosomal syntaxin and the AP-3 complex are required for formation and maturation of candidate lysosome-related secretory organelles (mucocysts) in Tetrahymena thermophila.Mol Biol Cell. 2017 Jun 1;28(11):1551-1564. doi: 10.1091/mbc.E17-01-0018. Epub 2017 Apr 5. Mol Biol Cell. 2017. PMID: 28381425 Free PMC article.
-
Diversification of CORVET tethers facilitates transport complexity in Tetrahymena thermophila.J Cell Sci. 2020 Feb 12;133(3):jcs238659. doi: 10.1242/jcs.238659. J Cell Sci. 2020. PMID: 31964712 Free PMC article.
-
Distinct sets of tethering complexes, SNARE complexes, and Rab GTPases mediate membrane fusion at the vacuole in Arabidopsis.Proc Natl Acad Sci U S A. 2018 Mar 6;115(10):E2457-E2466. doi: 10.1073/pnas.1717839115. Epub 2018 Feb 20. Proc Natl Acad Sci U S A. 2018. PMID: 29463724 Free PMC article.
-
Tethering complexes in the endocytic pathway: CORVET and HOPS.FEBS J. 2013 Jun;280(12):2743-57. doi: 10.1111/febs.12151. Epub 2013 Feb 21. FEBS J. 2013. PMID: 23351085 Review.
-
CORVET and HOPS tethering complexes - coordinators of endosome and lysosome fusion.J Cell Sci. 2013 Mar 15;126(Pt 6):1307-16. doi: 10.1242/jcs.107805. J Cell Sci. 2013. PMID: 23645161 Review.
Cited by
-
Ancient complement and lineage-specific evolution of the Sec7 ARF GEF proteins in eukaryotes.Mol Biol Cell. 2019 Jul 15;30(15):1846-1863. doi: 10.1091/mbc.E19-01-0073. Epub 2019 May 29. Mol Biol Cell. 2019. PMID: 31141460 Free PMC article.
-
The road to lysosome-related organelles: Insights from Hermansky-Pudlak syndrome and other rare diseases.Traffic. 2019 Jun;20(6):404-435. doi: 10.1111/tra.12646. Traffic. 2019. PMID: 30945407 Free PMC article. Review.
-
Tetrahymena ATG8 homologs, TtATG8A and TtATG8B, are responsible for mitochondrial degradation induced by starvation.mBio. 2025 Jun 11;16(6):e0078325. doi: 10.1128/mbio.00783-25. Epub 2025 May 15. mBio. 2025. PMID: 40372018 Free PMC article.
-
Assessing the utility of Hsp90 gene for inferring evolutionary relationships within the ciliate subclass Hypotricha (Protista, Ciliophora).BMC Evol Biol. 2020 Jul 16;20(1):86. doi: 10.1186/s12862-020-01653-0. BMC Evol Biol. 2020. PMID: 32677880 Free PMC article.
-
ESCargo: a regulatable fluorescent secretory cargo for diverse model organisms.Mol Biol Cell. 2020 Dec 15;31(26):2892-2903. doi: 10.1091/mbc.E20-09-0591. Epub 2020 Oct 28. Mol Biol Cell. 2020. PMID: 33112725 Free PMC article.
References
-
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:347–358. - PubMed
-
- Keller P, Simons K. Post-Golgi biosynthetic trafficking. J Cell Sci. 1997;110(Pt 24):3001–3009. - PubMed
-
- Orci L, Ravazzola M, Amherdt M, Perrelet A, Powell SK, Quinn DL, Moore HP. The trans-most cisternae of the Golgi complex: a compartment for sorting of secretory and plasma membrane proteins. Cell. 1987;51:1039–1051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

