Heterogeneity of SOX9 and HNF1β in Pancreatic Ducts Is Dynamic
- PMID: 29478894
- PMCID: PMC5918495
- DOI: 10.1016/j.stemcr.2018.01.028
Heterogeneity of SOX9 and HNF1β in Pancreatic Ducts Is Dynamic
Abstract
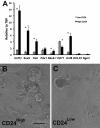
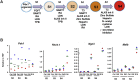
Pancreatic duct epithelial cells have been suggested as a source of progenitors for pancreatic growth and regeneration. However, genetic lineage-tracing experiments with pancreatic duct-specific Cre expression have given conflicting results. Using immunofluorescence and flow cytometry, we show heterogeneous expression of both HNF1β and SOX9 in adult human and murine ductal epithelium. Their expression was dynamic and diminished significantly after induced replication. Purified pancreatic duct cells formed organoid structures in 3D culture, and heterogeneity of expression of Hnf1β and Sox9 was maintained even after passaging. Using antibodies against a second cell surface molecule CD51 (human) or CD24 (mouse), we could isolate living subpopulations of duct cells enriched for high or low expression of HNF1β and SOX9. Only the CD24high (Hnfβhigh/Sox9high) subpopulation was able to form organoids.
Keywords: heterogeneity; organoid; pancreatic ductal cells.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Inversin-deficient (inv) mice do not establish a polarized duct system in the liver and pancreas.Anat Rec (Hoboken). 2024 Jun;307(6):2197-2212. doi: 10.1002/ar.25346. Epub 2023 Nov 3. Anat Rec (Hoboken). 2024. PMID: 37921502
-
Sox9EGFP Defines Biliary Epithelial Heterogeneity Downstream of Yap Activity.Cell Mol Gastroenterol Hepatol. 2021;11(5):1437-1462. doi: 10.1016/j.jcmgh.2021.01.009. Epub 2021 Jan 23. Cell Mol Gastroenterol Hepatol. 2021. PMID: 33497866 Free PMC article.
-
NFATc1 Links EGFR Signaling to Induction of Sox9 Transcription and Acinar-Ductal Transdifferentiation in the Pancreas.Gastroenterology. 2015 May;148(5):1024-1034.e9. doi: 10.1053/j.gastro.2015.01.033. Epub 2015 Jan 23. Gastroenterology. 2015. PMID: 25623042 Free PMC article.
-
Sox9 and programming of liver and pancreatic progenitors.J Clin Invest. 2013 May;123(5):1881-6. doi: 10.1172/JCI66022. Epub 2013 May 1. J Clin Invest. 2013. PMID: 23635786 Free PMC article. Review.
-
Can pancreatic duct-derived progenitors be a source of islet regeneration?Biochem Biophys Res Commun. 2009 Jun 12;383(4):383-5. doi: 10.1016/j.bbrc.2009.03.114. Epub 2009 Mar 24. Biochem Biophys Res Commun. 2009. PMID: 19324022 Review.
Cited by
-
Aldh1b1 expression defines progenitor cells in the adult pancreas and is required for Kras-induced pancreatic cancer.Proc Natl Acad Sci U S A. 2019 Oct 8;116(41):20679-20688. doi: 10.1073/pnas.1901075116. Epub 2019 Sep 23. Proc Natl Acad Sci U S A. 2019. PMID: 31548432 Free PMC article.
-
Lfng-expressing centroacinar cell is a unique cell-of-origin for p53 deficient pancreatic cancer.Oncogene. 2025 Feb;44(6):348-362. doi: 10.1038/s41388-024-03226-7. Epub 2024 Nov 15. Oncogene. 2025. PMID: 39548190 Free PMC article.
-
Towards a better understanding of diabetes mellitus using organoid models.Nat Rev Endocrinol. 2023 Apr;19(4):232-248. doi: 10.1038/s41574-022-00797-x. Epub 2023 Jan 20. Nat Rev Endocrinol. 2023. PMID: 36670309 Free PMC article. Review.
-
Pancreatic Progenitors: There and Back Again.Trends Endocrinol Metab. 2019 Jan;30(1):4-11. doi: 10.1016/j.tem.2018.10.002. Epub 2018 Nov 28. Trends Endocrinol Metab. 2019. PMID: 30502039 Free PMC article. Review.
-
Sox9 inhibits Activin A to promote biliary maturation and branching morphogenesis.Nat Commun. 2025 Feb 15;16(1):1667. doi: 10.1038/s41467-025-56813-x. Nat Commun. 2025. PMID: 39955269 Free PMC article.
References
-
- Al-Hasani K., Pfeifer A., Courtney M., Ben-Othman N., Gjernes E., Vieira A., Druelle N., Avolio F., Ravassard P., Leuckx G. Adult duct-lining cells can reprogram into β-like cells able to counter repeated cycles of toxin-induced diabetes. Dev. Cell. 2013;26:86–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

