High-Yield Purification, Preservation, and Serial Transplantation of Human Satellite Cells
- PMID: 29478895
- PMCID: PMC5918346
- DOI: 10.1016/j.stemcr.2018.01.022
High-Yield Purification, Preservation, and Serial Transplantation of Human Satellite Cells
Abstract
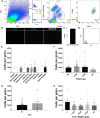
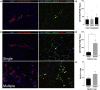
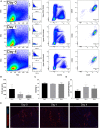
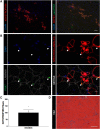
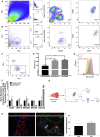
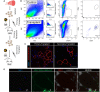
Investigation of human muscle regeneration requires robust methods to purify and transplant muscle stem and progenitor cells that collectively constitute the human satellite cell (HuSC) pool. Existing approaches have yet to make HuSCs widely accessible for researchers, and as a result human muscle stem cell research has advanced slowly. Here, we describe a robust and predictable HuSC purification process that is effective for each human skeletal muscle tested and the development of storage protocols and transplantation models in dystrophin-deficient and wild-type recipients. Enzymatic digestion, magnetic column depletion, and 6-marker flow-cytometric purification enable separation of 104 highly enriched HuSCs per gram of muscle. Cryostorage of HuSCs preserves viability, phenotype, and transplantation potential. Development of enhanced and species-specific transplantation protocols enabled serial HuSC xenotransplantation and recovery. These protocols and models provide an accessible system for basic and translational investigation and clinical development of HuSCs.
Keywords: human satellite cell purification; satellite cell cryopreservation; serial transplantation.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Engraftment of FACS Isolated Muscle Stem Cells into Injured Skeletal Muscle.Methods Mol Biol. 2017;1556:223-236. doi: 10.1007/978-1-4939-6771-1_11. Methods Mol Biol. 2017. PMID: 28247352
-
Ex Vivo Expansion and In Vivo Self-Renewal of Human Muscle Stem Cells.Stem Cell Reports. 2015 Oct 13;5(4):621-32. doi: 10.1016/j.stemcr.2015.08.004. Epub 2015 Sep 3. Stem Cell Reports. 2015. PMID: 26344908 Free PMC article.
-
Human Satellite Cell Isolation and Xenotransplantation.Methods Mol Biol. 2017;1668:105-123. doi: 10.1007/978-1-4939-7283-8_8. Methods Mol Biol. 2017. PMID: 28842905
-
Muscle-derived stem cells.Cell Cycle. 2004 Feb;3(2):104-7. Cell Cycle. 2004. PMID: 14712064 Review.
-
Stem and progenitor cells in skeletal muscle development, maintenance, and therapy.Mol Ther. 2007 May;15(5):867-77. doi: 10.1038/mt.sj.6300145. Epub 2007 Mar 27. Mol Ther. 2007. PMID: 17387336 Review.
Cited by
-
A Promising Future for Stem-Cell-Based Therapies in Muscular Dystrophies-In Vitro and In Vivo Treatments to Boost Cellular Engraftment.Int J Mol Sci. 2019 Oct 31;20(21):5433. doi: 10.3390/ijms20215433. Int J Mol Sci. 2019. PMID: 31683627 Free PMC article. Review.
-
Human Tissue-Engineered Skeletal Muscle: A Tool for Metabolic Research.Endocrinol Metab (Seoul). 2022 Jun;37(3):408-414. doi: 10.3803/EnM.2022.302. Epub 2022 Jun 29. Endocrinol Metab (Seoul). 2022. PMID: 35798547 Free PMC article.
-
Defining the Skeletal Myogenic Lineage in Human Pluripotent Stem Cell-Derived Teratomas.Cells. 2022 May 9;11(9):1589. doi: 10.3390/cells11091589. Cells. 2022. PMID: 35563894 Free PMC article.
-
Purification and preservation of satellite cells from human skeletal muscle.STAR Protoc. 2021 Jan 29;2(1):100302. doi: 10.1016/j.xpro.2021.100302. eCollection 2021 Mar 19. STAR Protoc. 2021. PMID: 33554137 Free PMC article.
-
Myoblast deactivation within engineered human skeletal muscle creates a transcriptionally heterogeneous population of quiescent satellite-like cells.Biomaterials. 2022 May;284:121508. doi: 10.1016/j.biomaterials.2022.121508. Epub 2022 Apr 7. Biomaterials. 2022. PMID: 35421801 Free PMC article.
References
-
- Brimah K., Ehrhardt J., Mouly V., Butler-Browne G.S., Partridge T.A., Morgan J.E. Human muscle precursor cell regeneration in the mouse host is enhanced by growth factors. Hum. Gene Ther. 2004;15:1109–1124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

