Distinct roles for phosphoinositide 3-kinases γ and δ in malignant B cell migration
- PMID: 29479062
- PMCID: PMC6127087
- DOI: 10.1038/s41375-018-0012-5
Distinct roles for phosphoinositide 3-kinases γ and δ in malignant B cell migration
Abstract
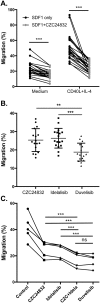
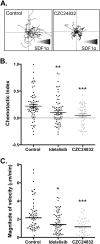
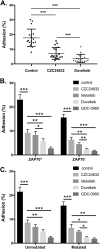
The PI 3-kinases (PI3K) are essential mediators of chemokine receptor signaling necessary for migration of chronic lymphocytic leukemia (CLL) cells and their interaction with tissue-resident stromal cells. While the PI3Kδ-specific inhibitor idelalisib shows efficacy in treatment of CLL and other B cell malignancies, the function of PI3Kγ has not been extensively studied in B cells. Here, we assess whether PI3Kγ has non-redundant functions in CLL migration and adhesion to stromal cells. We observed that pharmaceutical PI3Kγ inhibition with CZC24832 significantly impaired CLL cell migration, while dual PI3Kδ/γ inhibitor duvelisib had a greater impact than single isoform-selective inhibitors. Knockdown of PI3Kγ reduced migration of CLL cells and cell lines. Expression of the PI3Kγ subunits increased in CLL cells in response to CD40L/IL-4, whereas BCR cross-linking had no effect. Overexpression of PI3Kγ subunits enhanced cell migration in response to SDF1α/CXCL12, with the strongest effect observed within ZAP70 + CLL samples. Microscopic tracking of cell migration within chemokine gradients revealed that PI3Kγ functions in gradient sensing and impacts cell morphology and F-actin polarization. PI3Kγ inhibition also reduced CLL adhesion to stromal cells to a similar extent as idelalisib. These findings provide the first evidence that PI3Kγ has unique functions in malignant B cells.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Lagneaux L, Delforge A, Bron D, De Bruyn C, Stryckmans P. Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood. 1998;91:2387–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

