The spread and evolution of rabies virus: conquering new frontiers
- PMID: 29479072
- PMCID: PMC6899062
- DOI: 10.1038/nrmicro.2018.11
The spread and evolution of rabies virus: conquering new frontiers
Abstract
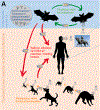
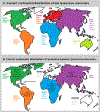
Rabies is a lethal zoonotic disease that is caused by lyssaviruses, most often rabies virus. Despite control efforts, sporadic outbreaks in wildlife populations are largely unpredictable, underscoring our incomplete knowledge of what governs viral transmission and spread in reservoir hosts. Furthermore, the evolutionary history of rabies virus and related lyssaviruses remains largely unclear. Robust surveillance efforts combined with diagnostics and disease modelling are now providing insights into the epidemiology and evolution of rabies virus. The immune status of the host, the nature of exposure and strain differences all clearly influence infection and transmission dynamics. In this Review, we focus on rabies virus infections in the wildlife and synthesize current knowledge in the rapidly advancing fields of rabies virus epidemiology and evolution, and advocate for multidisciplinary approaches to advance our understanding of this disease.
Conflict of interest statement
There is
Figures



References
-
- Baer GM The natural history of rabies. (CRC press, 1991).
-
- Yuhong W Rabies and rabid dogs in Sumerian and Akkadian literature. Journal of the American Oriental Society, 32–43 (2001).
-
- Baer GM in Rabies (ed Wunner William H Jackson Alan C) Ch. 1, 1–22 (2007).
-
- Belotto A, Leanes L, Schneider M, Tamayo H & Correa E Overview of rabies in the Americas. Virus research 111, 5–12 (2005). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

