Adrenergic Signaling: A Targetable Checkpoint Limiting Development of the Antitumor Immune Response
- PMID: 29479349
- PMCID: PMC5812031
- DOI: 10.3389/fimmu.2018.00164
Adrenergic Signaling: A Targetable Checkpoint Limiting Development of the Antitumor Immune Response
Abstract
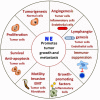
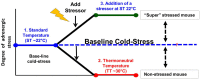
An immune response must be tightly controlled so that it will be commensurate with the level of response needed to protect the organism without damaging normal tissue. The roles of cytokines and chemokines in orchestrating these processes are well known, but although stress has long been thought to also affect immune responses, the underlying mechanisms were not as well understood. Recently, the role of nerves and, specifically, the sympathetic nervous system, in regulating immune responses is being revealed. Generally, an acute stress response is beneficial but chronic stress is detrimental because it suppresses the activities of effector immune cells while increasing the activities of immunosuppressive cells. In this review, we first discuss the underlying biology of adrenergic signaling in cells of both the innate and adaptive immune system. We then focus on the effects of chronic adrenergic stress in promoting tumor growth, giving examples of effects on tumor cells and immune cells, explaining the methods commonly used to induce stress in preclinical mouse models. We highlight how this relates to our observations that mandated housing conditions impose baseline chronic stress on mouse models, which is sufficient to cause chronic immunosuppression. This problem is not commonly recognized, but it has been shown to impact conclusions of several studies of mouse physiology and mouse models of disease. Moreover, the fact that preclinical mouse models are chronically immunosuppressed has critical ramifications for analysis of any experiments with an immune component. Our group has found that reducing adrenergic stress by housing mice at thermoneutrality or treating mice housed at cooler temperatures with β-blockers reverses immunosuppression and significantly improves responses to checkpoint inhibitor immunotherapy. These observations are clinically relevant because there are numerous retrospective epidemiological studies concluding that cancer patients who were taking β-blockers have better outcomes. Clinical trials testing whether β-blockers can be repurposed to improve the efficacy of traditional and immunotherapies in patients are on the horizon.
Keywords: adrenergic; antitumor immune response; norepinephrine; stress; temperature; β-blocker.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

