A Rice CPYC-Type Glutaredoxin OsGRX20 in Protection against Bacterial Blight, Methyl Viologen and Salt Stresses
- PMID: 29479359
- PMCID: PMC5811478
- DOI: 10.3389/fpls.2018.00111
A Rice CPYC-Type Glutaredoxin OsGRX20 in Protection against Bacterial Blight, Methyl Viologen and Salt Stresses
Abstract
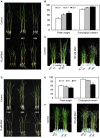
Glutaredoxins (GRXs) belong to the antioxidants involved in the cellular stress responses. In spite of the identification 48 GRX genes in rice genomes, the biological functions of most of them remain unknown. Especially, the biological roles of members of GRX family in disease resistance are still lacking. Our proteomic analysis found that OsGRX20 increased by 2.7-fold after infection by bacterial blight. In this study, we isolated and characterized the full-length nucleotide sequences of the rice OsGRX20 gene, which encodes a GRX family protein with CPFC active site of CPYC-type class. OsGRX20 protein was localized in nucleus and cytosol, and its transcripts were expressed predominantly in leaves. Several stress- and hormone-related motifs putatively acting as regulatory elements were found in the OsGRX20 promoter. Real-time quantitative PCR analysis indicated that OsGRX20 was expressed at a significantly higher level in leaves of a resistant or tolerant rice genotype, Yongjing 50A, than in a sensitive genotype, Xiushui 11, exposed to bacterial blight, methyl viologen, heat, and cold. Its expression could be induced by salt, PEG-6000, 2,4-D, salicylic acid, jasmonic acid, and abscisic acid treatments in Yongjing 50A. Overexpression of OsGRX20 in rice Xiushui 11 significantly enhanced its resistance to bacterial blight attack, and tolerance to methyl viologen and salt stresses. In contrast, interference of OsGRX20 in Yongjing 50A led to increased susceptibility to bacterial blight, methyl viologen and salt stresses. OsGRX20 restrained accumulation of superoxide radicals in aerial tissue during methyl viologen treatment. Consistently, alterations in OsGRX20 expression affect the ascorbate/dehydroascorbate ratio and the abundance of transcripts encoding four reactive oxygen species scavenging enzymes after methyl viologen-induced stress. Our results demonstrate that OsGRX20 functioned as a positive regulator in rice tolerance to multiple stresses, which may be of significant use in the genetic improvement of rice resistance.
Keywords: OsGRX20; bacterial blight; genetic transformation; glutaredoxin; methyl viologen; rice; salt stress.
Figures







References
LinkOut - more resources
Full Text Sources
Other Literature Sources

