Dysbiosis of gut microbiota in promoting the development of colorectal cancer
- PMID: 29479437
- PMCID: PMC5806407
- DOI: 10.1093/gastro/gox031
Dysbiosis of gut microbiota in promoting the development of colorectal cancer
Abstract
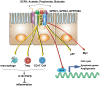
Gastrointestinal microbiome, containing at least 100 trillion bacteria, resides in the mucosal surface of human intestine. Recent studies show that perturbations in the microbiota may influence physiology and link to a number of diseases, including colon tumorigenesis. Colorectal cancer (CRC), the third most common cancer, is the disease resulting from multi-genes and multi-factors, but the mechanistic details between gut microenvironment and CRC remain poorly characterized. Thanks to new technologies such as metagenome sequencing, progress in large-scale analysis of the genetic and metabolic profile of gut microbial has been possible, which has facilitated studies about microbiota composition, taxonomic alterations and host interactions. Different bacterial species and their metabolites play critical roles in the development of CRC. Also, microbiota is important in the inflammatory response and immune processes deregulation during the development and progression of CRC. This review summarizes current studies regarding the association between gastrointestinal microbiota and the development of CRC, which provides insights into the therapeutic strategy of CRC.
Keywords: colorectal cancer; gut microbiota; microbiome dysbiosis; tumorigenesis.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

