Palladium-Catalyzed Decarbonylative Trifluoromethylation of Acid Fluorides
- PMID: 29479784
- PMCID: PMC5900963
- DOI: 10.1002/anie.201800644
Palladium-Catalyzed Decarbonylative Trifluoromethylation of Acid Fluorides
Abstract
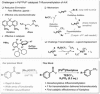
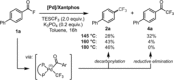
While acid fluorides can readily be made from widely available or biomass-feedstock-derived carboxylic acids, their use as functional groups in metal-catalyzed cross-coupling reactions is rare. This report presents the first demonstration of Pd-catalyzed decarbonylative functionalization of acid fluorides to yield trifluoromethyl arenes (ArCF3 ). The strategy relies on a Pd/Xantphos catalytic system and the supply of fluoride for transmetalation through intramolecular redistribution to the the Pd center. This strategy eliminated the need for exogenous and detrimental fluoride additives and allows Xantphos to be used in catalytic trifluoromethylations for the first time. Our experimental and computational mechanistic data support a sequence in which transmetalation by R3 SiCF3 occurs prior to decarbonylation.
Keywords: carboxylic acids; catalysis; density functional calculations; palladium; trifluoromethylation.
© 2018 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- For selected recent examples on decarbonylative and decarboxylative cross couplings, see:
-
- Takise R., Isshiki R., Muto K., Itami K., Yamaguchi J., J. Am. Chem. Soc. 2017, 139, 3340; - PubMed
-
- Guo L., Chatupheeraphat A., Rueping M., Angew. Chem. Int. Ed. 2016, 55, 11810; - PubMed
- Angew. Chem. 2016, 128, 11989;
-
- Shi S., Meng G., Szostak M., Angew. Chem. Int. Ed. 2016, 55, 6959; - PubMed
- Angew. Chem. 2016, 128, 7073;
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

