Preferential modulation of the lateral habenula activity by serotonin-2A rather than -2C receptors: Electrophysiological and neuroanatomical evidence
- PMID: 29479825
- PMCID: PMC6490100
- DOI: 10.1111/cns.12830
Preferential modulation of the lateral habenula activity by serotonin-2A rather than -2C receptors: Electrophysiological and neuroanatomical evidence
Abstract
Aims: Serotonergic (5-HT) modulation of the lateral habenula (LHb) activity is central in normal and pathologic conditions such as mood disorders. Among the multiple 5-HT receptors (5-HTRs) involved, the 5-HT2C R seems to play a pivotal role. Yet, the role of 5-HT2A Rs in the control of the LHb neuronal activity is completely unknown.
Methods: Single-cell extracellular recording of the LHb neurons was used in rats to study the effect of the general activation and blockade of the 5-HT2C R and 5-HT2A R with Ro 60-0175 and SB242084, TCB-2 and MDL11939, respectively. The expression of both receptors in the LHb was confirmed using immunohistochemistry.
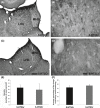
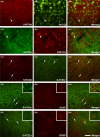
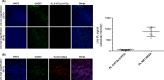
Results: Cumulative doses (5-640 μg/kg, iv) of Ro 60-0175 and TCB-2 affected the activity of 34% and 63% of the LHb recorded neurons, respectively. LHb neurons were either inhibited at low doses or excited at higher doses of the 5-HT2A/C R agonists. SB242084 or MDL11939 (both at 200 μg/kg, iv) did not modify neuronal firing when injected alone, but reverted the bidirectional effects of Ro 60-0175 or TCB-2, respectively. 5-HT2C Rs and 5-HT2A Rs are expressed in less than the 20% of the LHb neurons, and they neither colocalize nor make heterodimers. Strikingly, only 5-HT2A Rs are expressed by the majority of LHb astrocyte cells.
Conclusions: Peripheral administration of 5-HT2A R agonist promotes a heterogeneous pattern of neuronal responses in the LHb, and these effects are more prominent than those induced by the 5-HT2C R activation.
Keywords: addiction; depression; immunohistochemistry; lateral habenula; serotonin.
© 2018 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Acute and Chronic Nicotine Exposures Differentially Affect Central Serotonin 2A Receptor Function: Focus on the Lateral Habenula.Int J Mol Sci. 2020 Mar 9;21(5):1873. doi: 10.3390/ijms21051873. Int J Mol Sci. 2020. PMID: 32182934 Free PMC article.
-
Activation of serotonin(2C) receptors in the lateral habenular nucleus increases the expression of depression-related behaviors in the hemiparkinsonian rat.Neuropharmacology. 2015 Jun;93:68-79. doi: 10.1016/j.neuropharm.2015.01.024. Epub 2015 Feb 7. Neuropharmacology. 2015. PMID: 25661701
-
Dorsal raphe projection inhibits the excitatory inputs on lateral habenula and alleviates depressive behaviors in rats.Brain Struct Funct. 2018 Jun;223(5):2243-2258. doi: 10.1007/s00429-018-1623-3. Epub 2018 Feb 19. Brain Struct Funct. 2018. PMID: 29460052
-
The lateral habenula and the serotonergic system.Pharmacol Biochem Behav. 2017 Nov;162:22-28. doi: 10.1016/j.pbb.2017.05.007. Epub 2017 May 17. Pharmacol Biochem Behav. 2017. PMID: 28528079 Review.
-
Synaptic and cellular profile of neurons in the lateral habenula.Front Hum Neurosci. 2013 Dec 16;7:860. doi: 10.3389/fnhum.2013.00860. Front Hum Neurosci. 2013. PMID: 24379770 Free PMC article. Review.
Cited by
-
Preferential Modulatory Action of 5-HT2A Receptors on the Dynamic Regulation of Basal Ganglia Circuits.J Neurosci. 2023 Jan 4;43(1):56-67. doi: 10.1523/JNEUROSCI.1181-22.2022. Epub 2022 Nov 18. J Neurosci. 2023. PMID: 36400530 Free PMC article.
-
Acute and Chronic Nicotine Exposures Differentially Affect Central Serotonin 2A Receptor Function: Focus on the Lateral Habenula.Int J Mol Sci. 2020 Mar 9;21(5):1873. doi: 10.3390/ijms21051873. Int J Mol Sci. 2020. PMID: 32182934 Free PMC article.
-
Lateral Habenula 5-HT2C Receptor Function Is Altered by Acute and Chronic Nicotine Exposures.Int J Mol Sci. 2021 Apr 30;22(9):4775. doi: 10.3390/ijms22094775. Int J Mol Sci. 2021. PMID: 33946328 Free PMC article.
-
Multi-level variations of lateral habenula in depression: A comprehensive review of current evidence.Front Psychiatry. 2022 Oct 28;13:1043846. doi: 10.3389/fpsyt.2022.1043846. eCollection 2022. Front Psychiatry. 2022. PMID: 36386995 Free PMC article. Review.
-
Expression of serotonin 1A and 2A receptors in molecular- and projection-defined neurons of the mouse insular cortex.Mol Brain. 2020 Jun 29;13(1):99. doi: 10.1186/s13041-020-00605-5. Mol Brain. 2020. PMID: 32594910 Free PMC article.
References
-
- Metzger M, Bueno D, Lima LB. The lateral habenula and the serotonergic system. Pharmacol Biochem Behav. 2017;162:22‐28. - PubMed
-
- De Deurwaerdere P, Di Giovanni G. Serotonergic modulation of the activity of mesencephalic dopaminergic systems: therapeutic implications. Prog Neurobiol. 2017;151:175‐236. - PubMed
-
- Millan MJ. Serotonin 5‐HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies. Therapie. 2005;60:441‐460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

