A hierarchical prognostic model for risk stratification in patients with early breast cancer according to 18 F-fludeoxyglucose uptake and clinicopathological parameters
- PMID: 29479851
- PMCID: PMC5911607
- DOI: 10.1002/cam4.1394
A hierarchical prognostic model for risk stratification in patients with early breast cancer according to 18 F-fludeoxyglucose uptake and clinicopathological parameters
Abstract
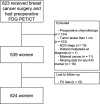
This study was to investigate a hierarchical prognostic model using clinicopathological factors and 18 F-fludeoxyglucose (FDG) uptake on positron emission tomography/computed tomography (PET/CT) for recurrence-free survival (RFS) in patients with early breast cancer who underwent surgery without neoadjuvant chemotherapy. A total of 524 patients with early breast cancer were included. The Cox proportional hazards model was used with clinicopathological variables and maximum standardized uptake value (SUVmax) on PET/CT. After classification and regression tree (CART) modeling, RFS curves were estimated using the Kaplan-Meier method and differences in each risk layer were assessed using the log-rank test. During a median follow-up of 46.2 months, 31 (5.9%) patients experienced recurrence. The CART model identified four risk layers: group 1 (SUVmax ≤6.75 and tumor size ≤2.0 cm); group 2 (SUVmax ≤6.75 and Luminal A [LumA] or TN tumor >2.0 cm); group 3 (SUVmax ≤6.75 and Luminal B [LumB] or human epidermal growth factor receptor 2 [HER2]-enriched] tumor >2.0 cm); group 4 (SUVmax >6.75). Five-year RFS was as follows: 95.9% (group 1), 98% (group 2), 82.8% (group 3), and 85.4% (group 4). Group 3 or group 4 showed worse prognosis than group 1 or group 2 (group 1 vs. group 3: P = 0.040; group 1 vs. group 4: P < 0.001; group 2 vs. group 3: P = 0.016; group 2 vs. group 4: P < 0.001). High SUVmax (>6.75) in primary breast cancer was an independent factor for poor RFS. In patients with low SUVmax, LumB or HER2-enriched tumor >2 cm was also prognostic for poor RFS, similar to high SUVmax.
Keywords: 18F-FDG PET/CT; classification and regression tree modeling; early breast cancer; recurrence-free survival; risk model.
© 2018 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures


Similar articles
-
The correlation of 18F-FDG PET/CT metabolic parameters, clinicopathological factors, and prognosis in breast cancer.Clin Transl Oncol. 2021 Mar;23(3):620-627. doi: 10.1007/s12094-020-02457-w. Epub 2020 Jul 18. Clin Transl Oncol. 2021. PMID: 32683540
-
Utility of (18)F FDG-PET/CT for predicting prognosis of luminal-type breast cancer.Breast Cancer Res Treat. 2015 Feb;150(1):209-17. doi: 10.1007/s10549-015-3303-9. Epub 2015 Feb 20. Breast Cancer Res Treat. 2015. PMID: 25697596 Free PMC article.
-
18FDG-PET/CT for predicting the outcome in ER+/HER2- breast cancer patients: comparison of clinicopathological parameters and PET image-derived indices including tumor texture analysis.Breast Cancer Res. 2017 Jan 5;19(1):3. doi: 10.1186/s13058-016-0793-2. Breast Cancer Res. 2017. PMID: 28057031 Free PMC article.
-
Prognostic significance of preoperative 18F-FDG PET/CT for breast cancer subtypes.Breast. 2016 Dec;30:5-12. doi: 10.1016/j.breast.2016.08.003. Epub 2016 Aug 29. Breast. 2016. PMID: 27569020
-
The prognostic value of SUVmax measuring on primary lesion and ALN by 18F-FDG PET or PET/CT in patients with breast cancer.Eur J Radiol. 2018 Aug;105:1-7. doi: 10.1016/j.ejrad.2018.05.014. Epub 2018 May 17. Eur J Radiol. 2018. PMID: 30017264 Review.
Cited by
-
Innovations in Positron Emission Tomography and State of the Art in the Evaluation of Breast Cancer Treatment Response.J Clin Med. 2023 Dec 27;13(1):154. doi: 10.3390/jcm13010154. J Clin Med. 2023. PMID: 38202160 Free PMC article. Review.
-
Prognostic value of the metabolic score obtained via [18F]FDG PET/CT and a new prognostic staging system for gastric cancer.Sci Rep. 2022 Nov 30;12(1):20681. doi: 10.1038/s41598-022-24877-0. Sci Rep. 2022. PMID: 36450778 Free PMC article.
-
Prognostic value of maximum standard uptake value, metabolic tumor volume, and total lesion glycolysis of positron emission tomography/computed tomography in patients with breast cancer: A systematic review and meta-analysis.PLoS One. 2019 Dec 11;14(12):e0225959. doi: 10.1371/journal.pone.0225959. eCollection 2019. PLoS One. 2019. PMID: 31826010 Free PMC article.
References
-
- Ferlay, J. , Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., et al. 2015. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136:E359–E386. - PubMed
-
- Bland, K. I. , Menck H. R., Scott‐Conner C. E., Morrow M., Winchester D. J., and Winchester D. P.. 1998. The National Cancer Data Base 10‐year survey of breast carcinoma treatment at hospitals in the United States. Cancer 83:1262–1273. - PubMed
-
- Zagouri, F. , Liakou P., Bartsch R., Peccatori F. A., Tsigginou A., Dimitrakakis C., et al. 2015. Discrepancies between ESMO and NCCN breast cancer guidelines: An appraisal. Breast (Edinburgh, Scotland). 24:513–523. - PubMed
-
- Gradishar, W. J. , Anderson B. O., Balassanian R., Blair S. L., Burstein H. J., Cyr A., et al. 2017. NCCN Guidelines Insights: Breast Cancer, Version 1.2017. J. Natl. Compr. Canc. Netw. 15:433–451. - PubMed
-
- Cianfrocca, M. , and Goldstein L. J.. 2004. Prognostic and predictive factors in early‐stage breast cancer. Oncologist 9:606–616. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

