Pridopidine: Overview of Pharmacology and Rationale for its Use in Huntington's Disease
- PMID: 29480206
- PMCID: PMC5836399
- DOI: 10.3233/JHD-170267
Pridopidine: Overview of Pharmacology and Rationale for its Use in Huntington's Disease
Abstract
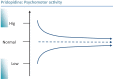
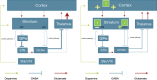
Despite advances in understanding the pathophysiology of Huntington's disease (HD), there are currently no effective pharmacological agents available to treat core symptoms or to stop or prevent the progression of this hereditary neurodegenerative disorder. Pridopidine, a novel small molecule compound, has demonstrated potential for both symptomatic treatment and disease modifying effects in HD. While pridopidine failed to achieve its primary efficacy outcomes (Modified motor score) in two trials (MermaiHD and HART) there were consistent effects on secondary outcomes (TMS). In the most recent study (PrideHD) pridiopidine did not differ from placebo on TMS, possibly due to a large enduring placebo effect.This review describes the process, based on in vivo systems response profiling, by which pridopidine was discovered and discusses its pharmacological profile, aiming to provide a model for the system-level effects, and a rationale for the use of pridopidine in patients affected by HD. Considering the effects on brain neurochemistry, gene expression and behaviour in vivo, pridopidine displays a unique effect profile. A hallmark feature in the behavioural pharmacology of pridopidine is its state-dependent inhibition or activation of dopamine-dependent psychomotor functions. Such effects are paralleled by strengthening of synaptic connectivity in cortico-striatal pathways suggesting pridopidine has potential to modify phenotypic expression as well as progression of HD. The preclinical pharmacological profile is discussed with respect to the clinical results for pridopidine, and proposals are made for further investigation, including preclinical and clinical studies addressing disease progression and effects at different stages of HD.
Keywords: Huntington’s disease; dopamine; dopamine D2 receptors; indirect pathway; motor control; prefrontal cortex; pridopidine; stabilizer; striatum.
Figures
References
-
- Martin JB, Gusella JF. Huntington’s disease. Pathogenesis and management. N Engl J Med. 1986;315(20):1267–76. - PubMed
-
- HDCRG. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72(6):971–83. - PubMed
-
- Krobitsch S, Kazantsev AG. Huntington’s disease: From molecular basis to therapeutic advances. Int J Biochem Cell Biol. 2011;43(1):20–4. - PubMed
-
- Goto S, Hirano A, Rojas-Corona RR. An immunohistochemical investigation of the human neostriatum in Huntington’s disease. Ann Neurol. 1989;25(3):298–304. - PubMed
-
- Graveland GA, Williams RS, DiFiglia M. Evidence for degenerative and regenerative changes in neostriatal spiny neurons in Huntington’s disease. Science. 1985;227(4688):770–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

