Decreased suicide rates in recent antidepressant clinical trials
- PMID: 29480436
- PMCID: PMC5920087
- DOI: 10.1007/s00213-018-4856-1
Decreased suicide rates in recent antidepressant clinical trials
Abstract
Rationale: The last systematic analysis of suicidality in antidepressant clinical trials submitted for approval by the US Food and Drug Administration was in 2000. Given the attention to suicide and antidepressants in the early 2000s, the authors aimed to evaluate if there have been any changes in suicide rates in antidepressant clinical trials following 2000.
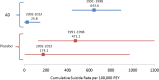
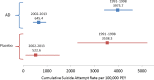
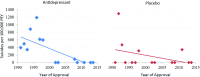
Objective and methods: The Integrated Safety Summary data from approval packets for 14 investigational antidepressant programs (1991-2013, 40,857 patients, 10,890.5 exposure years) were used to calculate suicides and suicide attempts per 100,000 patient exposure years (standardized rates) for antidepressant and placebo treatment groups separately. Suicides/suicide attempt rates, mean age, and percent female were compared between 1991 and 1998 (pre-2000) and 2002-2013 (post-2000). Drug-placebo differences in suicide/suicide attempt rates were explored.
Results: Among antidepressant-treated patients, the standardized suicide rate decreased significantly from pre- to post-2000 (643.6 to 25.8, p < 0.0001) as did the standardized suicide attempt rate (3975.7 to 645.4, p < 0.0001). For placebo-treated patients, the decrease was not significant for suicide rate (471.1 to 174.2, p = 0.66) but was significant for suicide attempt rate (from 3538.3 to 522.6, p < 0.001). Regression analysis showed a similar pattern with suicide/suicide attempt rates decreasing over time. None of the drug-placebo comparisons in suicide or suicide attempt rates were statistically significant. There was no change in percent female or mean age of patients in trials pre- and post-2000.
Conclusions: Deaths by suicide and suicide attempts have decreased significantly in antidepressant clinical trials following 2000 compared to the decade before 2000. Basic demographic features of the patients have remained consistent and medication treatment effects on suicidality were not apparent. These findings may reflect enhanced screening procedures and effective exclusion of suicidal patients in clinical trials for depression.
Keywords: Antidepressants; Clinical trials; Suicide; Suicide attempt.
Conflict of interest statement
Arif Khan, M.D., principal investigator of over 503 clinical trials sponsored by over 80 pharmaceutical companies and 30 CROs, has done neither compensated consulting nor speaking on their behalf, nor does he own stock in any of these or other pharmaceutical companies and therefore reports neither financial interest nor potential conflict of interest.
Kaysee Fahl Mar, MA, Sagarika Gokul, MBBS, and Dr. Brown report neither financial interest nor potential conflicts of interest.
Figures

References
-
- Bridge JA, Iyengar S, Salary C, Barbe RP, Birmaher B, Pincus HA, Ren L, Brent DA. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683–1696. doi: 10.1001/jama.297.15.1683. - DOI - PubMed
-
- Curtin SC, Warner M, Hedegaard H. Increase in suicide in the United States, 1999-2014. NCHS Data Brief. 2016;241:1–8. - PubMed
-
- FDA ACCESS DATA [database online]. Silver Spring, MD: Food and Drug Administration 2016. Updated November 11, 2016. http://www.accessdata.fda.gov/scripts/cder/daf/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

