Translational repression of HIF2α expression in mice with Chuvash polycythemia reverses polycythemia
- PMID: 29480820
- PMCID: PMC5873849
- DOI: 10.1172/JCI97684
Translational repression of HIF2α expression in mice with Chuvash polycythemia reverses polycythemia
Abstract
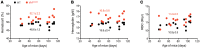
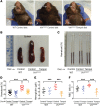
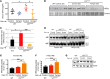
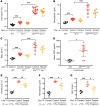
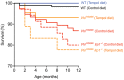
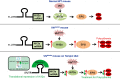
Chuvash polycythemia is an inherited disease caused by a homozygous germline VHLR200W mutation, which leads to impaired degradation of HIF2α, elevated levels of serum erythropoietin, and erythrocytosis/polycythemia. This phenotype is recapitulated by a mouse model bearing a homozygous VhlR200W mutation. We previously showed that iron-regulatory protein 1-knockout (Irp1-knockout) mice developed erythrocytosis/polycythemia through translational derepression of Hif2α, suggesting that IRP1 could be a therapeutic target to treat Chuvash polycythemia. Here, we fed VhlR200W mice supplemented with Tempol, a small, stable nitroxide molecule and observed that Tempol decreased erythropoietin production, corrected splenomegaly, normalized hematocrit levels, and increased the lifespans of these mice. We attribute the reversal of erythrocytosis/polycythemia to translational repression of Hif2α expression by Tempol-mediated increases in the IRE-binding activity of Irp1, as reversal of polycythemia was abrogated in VhlR200W mice in which Irp1 was genetically ablated. Thus, a new approach to the treatment of patients with Chuvash polycythemia may include dietary supplementation of Tempol, which decreased Hif2α expression and markedly reduced life-threatening erythrocytosis/polycythemia in the VhlR200W mice.
Keywords: Drug therapy; Hematology; Pharmacology; Translation.
Conflict of interest statement
Figures
Similar articles
-
Therapeutic inhibition of HIF-2α reverses polycythemia and pulmonary hypertension in murine models of human diseases.Blood. 2021 May 6;137(18):2509-2519. doi: 10.1182/blood.2020009138. Blood. 2021. PMID: 33512384 Free PMC article.
-
IRP1 regulates erythropoiesis and systemic iron homeostasis by controlling HIF2α mRNA translation.Blood. 2013 Aug 29;122(9):1658-68. doi: 10.1182/blood-2013-03-492454. Epub 2013 Jun 18. Blood. 2013. PMID: 23777768
-
Targeting HIF2α translation with Tempol in VHL-deficient clear cell renal cell carcinoma.Oncotarget. 2012 Nov;3(11):1472-82. doi: 10.18632/oncotarget.561. Oncotarget. 2012. PMID: 23178531 Free PMC article.
-
A Somatic HIF2α Mutation-Induced Multiple and Recurrent Pheochromocytoma/Paraganglioma with Polycythemia: Clinical Study with Literature Review.Endocr Pathol. 2017 Mar;28(1):75-82. doi: 10.1007/s12022-017-9469-4. Endocr Pathol. 2017. PMID: 28116635 Review.
-
HIF pathway mutations and erythrocytosis.Expert Rev Hematol. 2010 Feb;3(1):93-101. doi: 10.1586/ehm.09.68. Expert Rev Hematol. 2010. PMID: 21082936 Review.
Cited by
-
Toward a Novel Therapeutic Option for Polycythemia.Hemasphere. 2018 Jul 27;2(4):e139. doi: 10.1097/HS9.0000000000000139. eCollection 2018 Aug. Hemasphere. 2018. PMID: 31723788 Free PMC article. No abstract available.
-
An iron-sulfur cluster in the zinc-binding domain of the SARS-CoV-2 helicase modulates its RNA-binding and -unwinding activities.Proc Natl Acad Sci U S A. 2023 Aug 15;120(33):e2303860120. doi: 10.1073/pnas.2303860120. Epub 2023 Aug 8. Proc Natl Acad Sci U S A. 2023. PMID: 37552760 Free PMC article.
-
Fe-S cofactors in the SARS-CoV-2 RNA-dependent RNA polymerase are potential antiviral targets.Science. 2021 Jul 9;373(6551):236-241. doi: 10.1126/science.abi5224. Epub 2021 Jun 3. Science. 2021. PMID: 34083449 Free PMC article.
-
Hypoxia-inducible factor underlies von Hippel-Lindau disease stigmata.Elife. 2022 Aug 30;11:e80774. doi: 10.7554/eLife.80774. Elife. 2022. PMID: 36040300 Free PMC article.
-
Molecular Pathways Involved in the Development of Congenital Erythrocytosis.Genes (Basel). 2021 Jul 28;12(8):1150. doi: 10.3390/genes12081150. Genes (Basel). 2021. PMID: 34440324 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

