Restorative effects of human neural stem cell grafts on the primate spinal cord
- PMID: 29480894
- PMCID: PMC5922761
- DOI: 10.1038/nm.4502
Restorative effects of human neural stem cell grafts on the primate spinal cord
Abstract
We grafted human spinal cord-derived neural progenitor cells (NPCs) into sites of cervical spinal cord injury in rhesus monkeys (Macaca mulatta). Under three-drug immunosuppression, grafts survived at least 9 months postinjury and expressed both neuronal and glial markers. Monkey axons regenerated into grafts and formed synapses. Hundreds of thousands of human axons extended out from grafts through monkey white matter and synapsed in distal gray matter. Grafts gradually matured over 9 months and improved forelimb function beginning several months after grafting. These findings in a 'preclinical trial' support translation of NPC graft therapy to humans with the objective of reconstituting both a neuronal and glial milieu in the site of spinal cord injury.
Conflict of interest statement
The authors declare that they have no competing financial interests in this work.
Figures
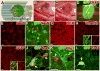
 and merged
and merged
 image in 5-month graft. (G)
image in 5-month graft. (G)
 (early neuronal marker) and (H)
(early neuronal marker) and (H)
 (early glial marker) were present in all grafts. (I)
(early glial marker) were present in all grafts. (I)
 (mature astrocyte marker) was only present in 5- and 9-month grafts (5 month graft shown). Vimentin and GFAP insets shown with and without
(mature astrocyte marker) was only present in 5- and 9-month grafts (5 month graft shown). Vimentin and GFAP insets shown with and without
 . (J)
. (J)
 (astrocyte and ependymal cell marker) was present in all grafts (arrows); at 5 and 9 months, 80% of these cells were astrocytes (GFAP+). (K)
(astrocyte and ependymal cell marker) was present in all grafts (arrows); at 5 and 9 months, 80% of these cells were astrocytes (GFAP+). (K)
 (oligodendrocyte precursor and immature oligodendrocyte marker) was present in all grafts (arrow) (L)
(oligodendrocyte precursor and immature oligodendrocyte marker) was present in all grafts (arrow) (L)
 (dividing cell marker) observed at low (1.5%) but detectable levels (arrow) at all time points. Scale bars: D, 1 mm; E–L, 10 μm. Panels E–F consist of five 0.5 μm confocal optical planes, Panels G, J, K, and L consist of two 0.5 μm confocal optical planes, and Panels H and I are single 0.5 μm confocal optical planes.
(dividing cell marker) observed at low (1.5%) but detectable levels (arrow) at all time points. Scale bars: D, 1 mm; E–L, 10 μm. Panels E–F consist of five 0.5 μm confocal optical planes, Panels G, J, K, and L consist of two 0.5 μm confocal optical planes, and Panels H and I are single 0.5 μm confocal optical planes.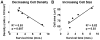

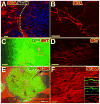
 ) regenerate short distances (up to 500 μm) into human NPC grafts. Dashed line indicates host/graft interface, revealed by
) regenerate short distances (up to 500 μm) into human NPC grafts. Dashed line indicates host/graft interface, revealed by
 labeling. (B) Sample axon 300 μm within graft, with complex branching pattern and bouton-like swellings. (C–D) Serotonergic (
labeling. (B) Sample axon 300 μm within graft, with complex branching pattern and bouton-like swellings. (C–D) Serotonergic (
 ) axons regenerate into human NPC grafts in all subjects with good rostral host-graft integration. (E–F) Host
) axons regenerate into human NPC grafts in all subjects with good rostral host-graft integration. (E–F) Host
 axons extensively regenerate into NPC grafts. Insets: Graft-derived GFP+ axons do not label for NF200, either within the graft or in host white matter (shown). Scale bars: A,B,D,F, 50 μm; C, 200 μm; E, 1 mm.
axons extensively regenerate into NPC grafts. Insets: Graft-derived GFP+ axons do not label for NF200, either within the graft or in host white matter (shown). Scale bars: A,B,D,F, 50 μm; C, 200 μm; E, 1 mm.Comment in
-
Spinal cord: Making new connections.Nat Rev Neurosci. 2018 May;19(5):252-253. doi: 10.1038/nrn.2018.33. Epub 2018 Mar 22. Nat Rev Neurosci. 2018. PMID: 29563574 No abstract available.
-
Transplanted neural progenitors bridge gaps to benefit cord-injured monkeys.Nat Med. 2018 Apr 10;24(4):388-390. doi: 10.1038/nm.4531. Nat Med. 2018. PMID: 29634690 Free PMC article. No abstract available.
References
-
- Blesch A, Tuszynski MH. Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2009;32:41–7. - PubMed
-
- Fawcett JW. Overcoming inhibition in the damaged spinal cord. J Neurotrauma. 2006;23:371–83. - PubMed
-
- He Z, Koprivica V. The Nogo signaling pathway for regeneration block. Annu Rev Neurosci. 2004;27:341–68. - PubMed
-
- Schwab ME. Nogo and axon regeneration. Curr Opin Neurobiol. 2004;14:118–24. - PubMed

